Wikipedia:Reference desk/Archives/Science/2023 July 20
| Science desk | ||
|---|---|---|
| < July 19 | << Jun | July | Aug >> | July 21 > |
| Welcome to the Wikipedia Science Reference Desk Archives |
|---|
| The page you are currently viewing is a transcluded archive page. While you can leave answers for any questions shown below, please ask new questions on one of the current reference desk pages. |
July 20[edit]
Blackbody emission questions.[edit]
1. Almost everything with heat emits IR. Humans, animals, water. The biggest source is the sun and fire. But I'm trying to find out who doesn't emit IR in darkness. I suppose humans and animals emit IR in darkness, but not water?
2. As sunlight is 52-55% IR and causes water to emit IR, I'm also curious to know how we draw the line between Blackbody radiation, and fluorescence/phosphorescence, of IR. Things that absorb IR, and emit in deep-IR. The thing is, if water stops emitting IR as soon as darkness, then that is equivalent to fluorescence, and if water emits for another 10 seconds after darkness, that's equivalent to phosphorescence.
3. They say most things that Blackbody IR at room temperature, will emit light, starting at red light, at 500 C. But what material is that, I've never found a chart for different materials such as steel, plastic, water (albeit some will turn liquid or gas 1st). What are some Blackbodys that have the lowest temperature to emit visible light? Thanks. 170.76.231.162 (talk) 16:17, 20 July 2023 (UTC).
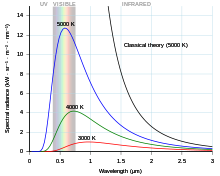
- All objects emit light, so long as they have a temperature, so ANY thing with a higher temperature than absolute zero will emit light. The specific spectrum of light that object emits is basically dependent only on the temperature, so long as the only source of light is from thermal radiation (there are OTHER processes that will emit light as well; the point of a blackbody is that it is an idealized object that can only emit thermal radiation. Simply put, all objects with any temperature at all emit thermal radiation; that thermal radiation (light wavelengths) fill follow a type of
normal distributionprobability distribution edit: The previous term was corrected from the original writing, which used an incorrect term. --Jayron32 11:05, 21 July 2023 (UTC) whose central limit is dependent on the temperature. In simple terms, the frequency distribution of the light will have the shape of a curve shown at right, with frequency on the X axis and intensity on the Y axis; the peak of the curve will be shifted further right for hotter objects and left for cooler objects. As some examples of this temperature dependence, humans have a temperature on order of about 300ish K, which corresponds to a frequency distribution whose peak is in the infrared. However, something like a lightbulb filament has a temperature on order of about 5000 K, which corresponds to an peak frequency somewhere near the middle of the visible range. If you look at the cosmic microwave background, which is basically the "temperature of space", it's about 3 K, which corresponds to a frequency peak in the (you guessed it) microwave range. However, it's important to note that this is a distribution and not a singular monochromatic light. The exact distribution (from which you could work out the relative amounts of each frequency of emitted light) is given by Planck's law. --Jayron32 16:35, 20 July 2023 (UTC)- Excellent answer, except for the bit about the normal distribution, which seems out of place here. Did you mean to refer to a more general term, like probability distribution? --Wrongfilter (talk) 10:03, 21 July 2023 (UTC)
- Yes, I chose the wrong term. Thank you for the correction. I have fixed it. --Jayron32 11:05, 21 July 2023 (UTC)
- I would drop any reference to the Central limit theorem that is not useful here where the simple expression "peak (of the curve)" is well understood. Philvoids (talk) 16:46, 21 July 2023 (UTC)
- Yes, I chose the wrong term. Thank you for the correction. I have fixed it. --Jayron32 11:05, 21 July 2023 (UTC)
- Excellent answer, except for the bit about the normal distribution, which seems out of place here. Did you mean to refer to a more general term, like probability distribution? --Wrongfilter (talk) 10:03, 21 July 2023 (UTC)
- I have no idea how to calculate this, but there must be a minimum yet greater than zero Kelvin temperature which under it no light will be emitted. A single photon of the longest wave light (deep red) has a fixed energy. When the energy of light is under that threshold, no light will be emitted. Zarnivop (talk) 17:21, 20 July 2023 (UTC)
- Not necessarily. The limit is set by Planck's constant, which merely states that the smallest unit of light is the photon, but a single photon can have any arbitrarily small frequency of light. E = hν, so for any frequency of light, the smallest amount of energy for that frequency is just Planck's constant times the frequency, but there is no lower limit on frequency. "Deep red" is still very high frequency light, with wavelengths on the order of a micrometer (1 millionth of a meter) or so. The CMB light has wavelengths of cm scale, however meter-length wavelengths or longer are perfectly common. VHF frequency radio waves have wavelengths in the meter-to-tens of meters range, while light with wavelengths as long as hundreds of thousands of kilometers (see ELF) which have been used to practical effect by humans. There is no functional "longest wavelength of light". There is a functional "Smallest quantum of energy for a given wavelength" (the photon) but that's not the same thing. --Jayron32 17:37, 20 July 2023 (UTC)
- Also, the light is emitted by a system of atoms, not by a single atom. If you've got 1023 atoms forming a solid with a temperature of a few kelvin, there's more than enough thermal energy to emit a single visible photon. It's not very likely to happen though. PiusImpavidus (talk) 19:33, 20 July 2023 (UTC)
- Light is by definition is bound between red (or at most IR) and purple (or at most UV). Radio frequencies are not light. Both are EM radiation, but you stated that anything above absolute 0 emits light. This is not true. EDIT: Anything above absolute zero emits photons, but not every photon is light - that depends on its energy. Zarnivop (talk) 22:27, 20 July 2023 (UTC)
- Engineering may have such a definition, but physics does not. In astronomy, we usually refer to ultraviolet and infrared radiation as "light", as the detector technology is basically the same as for "visible light", but if the mood takes us then light can occasionally mean all of em radiation. --Wrongfilter (talk) 10:03, 21 July 2023 (UTC)
- That's a rather facile understanding of light, and does not represent actual reality. There's nothing especially different between red light and radio waves, at all, except for the rather unimportant distinctions made by human biology. Since we aren't discussing human biology in this discussion, such distinctions aren't relevant. Clearly, putting such arbitrary distinctions on the discussion does not help answer the question correctly, which is why it isn't appropriate to do so. --Jayron32 11:02, 21 July 2023 (UTC)
- Do astronomers refer to radio frequencies as "light"[citation needed], I can totally see them include UV and IR, but I doubt anything else like radio, be it "facial" or whatnot. Zarnivop (talk) 13:53, 21 July 2023 (UTC)
- "facetious"? It's probably used very rarely when talking about radio specifically, but when somebody says "We get most of our information on the universe in the form of light", I would naturally understand radio as part of that (but not gravitational waves or neutrinos). But I admit this is a personal impression and I'm not going to look for references. --Wrongfilter (talk) 14:41, 21 July 2023 (UTC)
- I believe Jayron32 means FACILE adj. too simple to deal with the difficulties of a real situation, arrived at without due care or effort; lacking depth. I am unlikely to tell my grandmother that her cellphone works by sending light beams, nor should she expect to be lectured to by her grandchild that any doubt on this matter is merely due to her unimportant arbitrary, unreal and incorrect biological shallowness. Wrongfilter and Zamivop's comments correctly and appropriately remind us that in the vernacular English understood by the majority of general readers, and by all English speakers before Maxwell's demonstration in 1865 of the electromagnetic field, the word "light" means principally visible light. I think we can all distinguish between RADAR and LIDAR. Philvoids (talk) 21:03, 21 July 2023 (UTC)
- "facetious"? It's probably used very rarely when talking about radio specifically, but when somebody says "We get most of our information on the universe in the form of light", I would naturally understand radio as part of that (but not gravitational waves or neutrinos). But I admit this is a personal impression and I'm not going to look for references. --Wrongfilter (talk) 14:41, 21 July 2023 (UTC)
- Do astronomers refer to radio frequencies as "light"[citation needed], I can totally see them include UV and IR, but I doubt anything else like radio, be it "facial" or whatnot. Zarnivop (talk) 13:53, 21 July 2023 (UTC)
- Not necessarily. The limit is set by Planck's constant, which merely states that the smallest unit of light is the photon, but a single photon can have any arbitrarily small frequency of light. E = hν, so for any frequency of light, the smallest amount of energy for that frequency is just Planck's constant times the frequency, but there is no lower limit on frequency. "Deep red" is still very high frequency light, with wavelengths on the order of a micrometer (1 millionth of a meter) or so. The CMB light has wavelengths of cm scale, however meter-length wavelengths or longer are perfectly common. VHF frequency radio waves have wavelengths in the meter-to-tens of meters range, while light with wavelengths as long as hundreds of thousands of kilometers (see ELF) which have been used to practical effect by humans. There is no functional "longest wavelength of light". There is a functional "Smallest quantum of energy for a given wavelength" (the photon) but that's not the same thing. --Jayron32 17:37, 20 July 2023 (UTC)
- I took upon myself to replace the image given here with the actual graph. The old graph accurately depicted that the maximum-emission wavelength changed with temperature, but (wrongly) implied that for a given wavelength, an increase of temperature could decrease the emission. That is not true - for all wavelengths, spectral emissivity increases with temperature. TigraanClick here for my talk page ("private" contact) 08:44, 21 July 2023 (UTC)
- On the original question it should be pointed out that fluorescence and phosphorescence are line emissions, light is emitted only at certain wavelengths/frequencies given by electronic transitions in atoms or molecules. Thermal emission on the other hand is continuous emission, i.e. light is emitted at any wavelength (in any real situation probably better described as quasi-continuous). These are rather different processes. --Wrongfilter (talk) 10:03, 21 July 2023 (UTC)
- It should be noted that bodies only emit radiation according to the black body curve if they are perfect absorbers. It is fundamental, arising from electron orbits jumping down on emission and jumping up on absorption that any body of material can only emit on wavelengths that it can absorb. Thus glass, which is transparent to most wavelengths, cannot emit - well, sort of, at high enough temperatures, glass looses its transparency and can there emit.
- Carbon is the most perfect black body radiator, so to answer your question, it will begin emitting visible light at the lowest temperature. But the difference between it and steel is not great. Steel emits visible light at much lower temperatures that metals such as aluminium - as you would expect, as aluminium is a good mirror - it doesn't absorb light very well, compared to steel. Dionne Court (talk) 02:21, 24 July 2023 (UTC)
- You're perfectly right to point out that no real material is truly black and that therefore their thermal emission lies below the curves for the ideal black body; the ratio is called the emissivity and it depends on wavelength. However, just as no material is truly black (emissivity 1), no material is perfectly transparent or reflective (emissivity 0; the table in emissivity puts pure nitrogen or oxygen gas at essentially zero). It therefore remains true that all bodies do emit thermal radiation, some at a lower level than others. If you're looking for a temperature at which a material begins emitting visible light, you have to set a threshold for the light emitted, e.g. by specifying how you want to measure that. --Wrongfilter (talk) 06:33, 24 July 2023 (UTC)
- It should also be noted that the "model" blackbody was actually an oven with a pinhole in it: See Black body#Cavity with a hole and Kirchhoff's law of thermal radiation#A hole in the wall of a cavity. This is what Gustav Kirchhoff used when he did his initial experiments on the subject. --Jayron32 16:07, 24 July 2023 (UTC)
- You're perfectly right to point out that no real material is truly black and that therefore their thermal emission lies below the curves for the ideal black body; the ratio is called the emissivity and it depends on wavelength. However, just as no material is truly black (emissivity 1), no material is perfectly transparent or reflective (emissivity 0; the table in emissivity puts pure nitrogen or oxygen gas at essentially zero). It therefore remains true that all bodies do emit thermal radiation, some at a lower level than others. If you're looking for a temperature at which a material begins emitting visible light, you have to set a threshold for the light emitted, e.g. by specifying how you want to measure that. --Wrongfilter (talk) 06:33, 24 July 2023 (UTC)
