Wikipedia:Reference desk/Archives/Science/2014 July 1
| Science desk | ||
|---|---|---|
| < June 30 | << Jun | July | Aug >> | July 2 > |
| Welcome to the Wikipedia Science Reference Desk Archives |
|---|
| The page you are currently viewing is an archive page. While you can leave answers for any questions shown below, please ask new questions on one of the current reference desk pages. |
July 1[edit]
Superconductor critical temperature graph[edit]
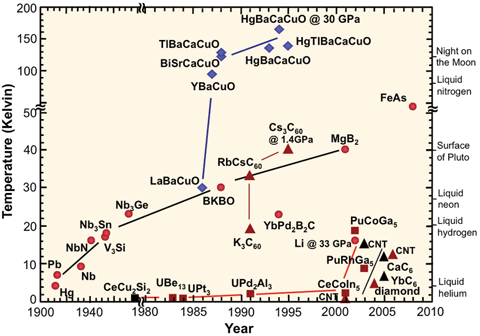
I would like to redraw File:Sc_history.gif to address the misleading axes (the year axis before 1980, and temperature axis above 50 K are compressed).
Could anyone familiar with the subject please tell me what the colours and shapes of the markers for each substance denote? I tried searching for a legend of the graphic online but couldn't find anything. It's no longer available at the original US government site.
Secondly, FeAs appears under 50 K in the top part of the graph, yet doesn't appear in the bottom part (which is under 50 K), so what is its critical temperature? Iron-based_superconductor lists many FeAs compounds but no plain FeAs.
Thanks, cmɢʟee⎆τaʟκ 19:17, 1 July 2014 (UTC)
- Not an answer, but rather a suggestion: If you redo that chart, I'd take the chemical formulas off the graph and place them in a legend, instead, and just put a number in place of each on the graph. This will make the graph less busy and easier to read. Also, ideally the legend would have links to the articles on each chemical. StuRat (talk) 23:30, 1 July 2014 (UTC)
- ..."easier to read" is often in the eye of the beholder. Replacing all the formulas with numbers means that at a single glance it is impossible to identify important compounds or chemical families. Someone wanting that sort of basic information would have to constantly shift their focus from the graph to the legend and back again. (Note, as well, that the "formulas" already present are, in many cases, abbreviated; for example, the XBaCuO and XCaCuO compounds are most assuredly not actually present in anything like 1:1:1:1 quantity.) Stripping out too much information can cause as much confusion as putting too much in.
- Arguably, one might be able to label the families of compounds in some cases (the blue diamonds are all YBCO-related structures, for instance) and add smaller notations for the individual family members, but there's limited gains to be made there. Some of the 'families' are pretty tenuously related. TenOfAllTrades(talk) 07:58, 2 July 2014 (UTC)
- I'd love to see the original legend for the categories. As I note above, the blue diamonds are YBCO/LBCO-related compounds. The dark red triangles are "3D"-carbon compounds (conjugates to carbon nanotubes or buckyballs, or diamond), the black triangles are "2D"-carbon compounds (intercalated graphite structures). The red circles seem to be mostly metals and simple binary compounds...except for the ones that aren't(?) The red squares are..."weird-ass actinide compounds"? Note that FeAs doesn't likely refer to a straight-up "iron arsenide" binary compound; presumably it is a catch-all for the ferropnictide [FeAs] layered structure, of which the best have (as you've seen in the linked article) critical temps just above 50 K.
- As for adjusting the vertical and horizontal scales, do be careful. You can linearize the vertical scale without too much harm if you're willing to accept a much taller figure; it works well if the point you want to convey is that the YBCO compounds were an astonishing leap forward in the field. If you try to keep the figure the same height, though, you squish together all the lower-Tc points. Similar crowding problems arise if you try to linearize the date axis. There are only eight points in the eighty years (one point per decade) between 1900 and 1980, whereas there are nearly thirty points in the subsequent thirty (almost one point per year). If the point you want to make is that the field was relatively quiet until the mid-1980s, that's fine; if you want the reader to be able to read approximate date and Tc information for particular compounds or classes...then completely linear date and Tc scales may not be as effective. TenOfAllTrades(talk) 07:58, 2 July 2014 (UTC)
- Looks like FeAs is ~54K, according to the introduction of [1].
- I would suggest trying out a semilog plot format for the Tc, since temperature practically wants a log before it anyway. Also a cool aspect of that plot is that you could have room temperature taunt the reader from the top of the graph. Wnt (talk) 14:45, 2 July 2014 (UTC)
- I second that suggestion for a semilog plot. This is a far more natural way to squeeze this information into a graph, compared to the current cut axes. The jumps in the axes essentially hide what the graph is trying to communicate, which is the progression in time. Crowding can be dealt with via fine lines from data points to the individual labels. —Quondum 16:13, 2 July 2014 (UTC)
Plastic in the Sun[edit]
I live in the Mojave Desert. I regularly find old plastic soda and water bottles out in the sand. Often, the bottles are so thin and brittle that the side facing up (in the sunlight) turns to dust when you touch it. This is, to me, degraded plastic. However, I am strongly into recycling and one common claim that I've read (and I use in my writings) is that it takes about 500 years for plastic to degrade. I know that these soda bottles are not anywhere near 500 years old. So, is this dust state of plastic not considered degraded plastic? If it is degraded, what is the justification for the standard "500 year" time for plastic degradation? I want to sound as though I have some knowledge of the matter because I'm sure others around here have seen plastic turned to dust as well. — Preceding unsigned comment added by 209.149.113.71 (talk) 19:54, 1 July 2014 (UTC)
- See the articles Biodegradeable and Biodegradable plastic for information. 84.209.89.214 (talk) 22:57, 1 July 2014 (UTC)
- The UV light causes this degradation in polymers such as plastics and rubber, unless they take precautions, such as adding dye to absorb the UV. This is why, incidentally, they quickly stopped making white rubber tires, as they rapidly degraded in sunlight, too. So, the 500 year time frame is for buried plastics, kept safe from UV light. StuRat (talk) 23:26, 1 July 2014 (UTC)
- Plastics degrade at differing rates depending on the composition of the material and on the environment it is in, such as exposure to UV. Some contain stabilizers to resist UV, others do not. The book "Green plastics" says (p53) that some polyolefins basically do not degrade, while more recently biodegradable plastics have been introduced which break down after use via the action of moisture, daylight, heat or biological activity. But I have seen tables which imply that it is bad to use plastic because it takes 500 uers to break down, without considering the composition and the environment it is exposed to. Edison (talk) 02:32, 2 July 2014 (UTC)
- I was too lazy to look it up but certainly "turning to dust" is not the same as degradation; see microbeads. :( Some of the "biodegradable" plastics of the past simply broke up into fibers. Of course, plastic will still degrade faster in the sun, while even newspapers might remain readable in landfills on a paleontological time scale. Wnt (talk) 14:37, 2 July 2014 (UTC)
- YES. I could take a new plastic bottle and run it through a shredder a few times, but it would not be decomposed, just broken into little pieces. As with microbeads, these small particles can still be potentially harmful. Though some depolymerization may occur in the desert sun over a few years, those small particles are still plastic, and in some ways even more harmful, since they can be easily dispersed and cannot be picked up and recycled the way an intact bottle can. I'd encourage the OP to tell friends and neighbors that picking up bottles before they disintegrate is the best option for avoiding environmental contamination. See also Plastic_particle_water_pollution and Microplastics. Most of the documented damage is in marine environments, but remember everything eventually gets washed to the sea. SemanticMantis (talk) 14:57, 2 July 2014 (UTC)
- Most Plastic is actually brittle in its raw form. That was a major problem with early plastic like Bakelite (1907). Today Plasticizers are used widely to put it in the state we are so familiar with that we take it for its oiginal state. Unfortunately these additives evapurate or degenetate rather fast over 20 - 30 years and much faster under exposure of Light, especially ultraviolet wavelength. So actually plastic "degenerates" much faster in 20-30 years. The 500 years are needed to decompose plastic completely. --Kharon (talk) 20:56, 2 July 2014 (UTC)
- The brittle plastics are thermoset plastics. Thermoplastics, on the other hand, despite the similar name, are soft and flexible at a certain temperature (typically room temperature), how we normally think of plastics. StuRat (talk) 21:19, 2 July 2014 (UTC)
- Good distinction. The problem that apparently none of us want to invest time in researching is: what exactly constitutes decomposition of a plastic? Small pieces of e.g. HDPE (commonly used for bottles) are still HDPE, and that can cause problems in both natural ecosystems, and in human health. When is HDPE no longer HDPE? It sounds like a koan, but that is the type of question we need to research to fully answer this question. I'm no good a this kind of detailed chemistry, but I suspect the long-chain molecules must be cut/broken until the constituent ingredients no longer have the physical and chemical properties of the parent plastic. UV light can apparently break such bonds, but that is similar to dilution -- how many drops of water must I add to my coca cola before it no longer coca cola? I think in the OP's description of bottles turning to dust some chains are broken, but many more remain. SemanticMantis (talk) 21:41, 2 July 2014 (UTC)
- The brittle plastics are thermoset plastics. Thermoplastics, on the other hand, despite the similar name, are soft and flexible at a certain temperature (typically room temperature), how we normally think of plastics. StuRat (talk) 21:19, 2 July 2014 (UTC)
- Thanks for all the comments. This sent me off to do more research. Water bottles are normally HDPE, which degrades quickly in UV light, and still degrades quickly without UV light. The 500 year statement is the average time for LDPE plastic to degrade. I found a nice chart that relates the recycle number to breakdown estimates. I don't feel that this negates my work in trying to limit plastic use. It helps focus on bad plastics and worse plastics. The site with the chart is http://www.brighthub.com/environment/green-living/articles/107380.aspx 209.149.113.71 (talk) 20:09, 3 July 2014 (UTC)
- Re. HDPE vs. LDPE: Note that HDPE molecules are practically linear, whereas LDPE molecules are highly branched. This has a TREMENDOUS impact on the rate of bacterial degradation, with linear molecules being degraded MUCH more readily than branched ones -- as we've already seen with synthetic detergents, which break down over time in water if the molecule is linear, but not if it's branched. 24.5.122.13 (talk) 22:21, 3 July 2014 (UTC)
