Wikipedia:Reference desk/Archives/Science/2012 September 12
| Science desk | ||
|---|---|---|
| < September 11 | << Aug | September | Oct >> | September 13 > |
| Welcome to the Wikipedia Science Reference Desk Archives |
|---|
| The page you are currently viewing is an archive page. While you can leave answers for any questions shown below, please ask new questions on one of the current reference desk pages. |
September 12[edit]
libya[edit]
Moved to Wikipedia:Reference_desk/Miscellaneous#libya - StuRat (talk) 01:14, 12 September 2012 (UTC)
Toothbrush disinfection[edit]
I tried bleach on my toothbrush to kill the germs, but, unfortunately, the bristles fell out. I'm considering trying either alcohol or hydrogen peroxide on my new toothbrush. So, will those also destroy my toothbrush ? StuRat (talk) 01:20, 12 September 2012 (UTC)
- I've used H2O2 (three percent) and do not recall it having any negative effects on the bristles, at least in a shorter time frame than you're supposed to replace it anyway. Of course I haven't actually tested whether it kills the germs. Also, I'd rinse it well before putting it in your mouth. --Trovatore (talk) 01:28, 12 September 2012 (UTC)
- Hydrogen peroxide is not very good as a disinfectant, as I understand it. Alcohol should do the trick, and I doubt it will cause much damage to the bristles, as opposed to bleach, which depending on the concentration is going to be a great deal more corrosive than alcohol. Evanh2008 (talk|contribs) 01:30, 12 September 2012 (UTC)
- Um, why are you worried about germs on your toothbrush? Unless you are putting it to unconventional use, the only place it is likely to get germs from is your mouth, and all you will be doing is putting them back (quite possibly dead) the next time you use it. Still, if you are that worried, according to WikiHow, who cite the American Dental Association, washing it under the tap both before and after use is sufficient most of the time, and you can place it inside a dishwasher occasionally for a deep cleanse: [1] I'd be wary of using a solvent like alcohol, as again the bristles might fall out, and neither bleach nor hydrogen peroxide (in germ-killing concentrations) sound particularly sensible substances to use on anything you intend to put in your mouth, even if they don't damage the toothbrush. AndyTheGrump (talk) 01:37, 12 September 2012 (UTC)
- One would suspect that if casual contamination or harm from germs in the bathroom was anywhere near a common occurrence to be a "serious concern" that it would 1. be something the ADA would mention it, 2. someone would sell a product for it, and 3. we'd all know people who routinely were injured in this fashion, probably including ourselves. I'm going to file this under "more things that StuRat worries about that nobody else does" unless there is some real scientific evidence of probability of harm. --Mr.98 (talk) 01:53, 12 September 2012 (UTC)
- Harm is not so clear, but contamination, yes. I'm not going to search for a cite from work, but this has been studied. Basically, flushing aerosolizes some of the contents of the toilet. Whether that's a problem depends, I suppose, on who shares the bathroom with you, and what they put in the toilet. --Trovatore (talk) 01:56, 12 September 2012 (UTC)
You can also put your tootbrush in the microwave. Count Iblis (talk) 01:41, 12 September 2012 (UTC)
- I'm not sure that would be a good idea. Apparently in some toothbrushes at least, the bristles are held in with tiny metal staples. Then again, I'm not sure that microwaving germs will necessarily kill them anyway - are you proposing to immerse the brush in water as you microwave it? AndyTheGrump (talk) 01:48, 12 September 2012 (UTC)
- (ec)Erm, technically this is a medical device, and suggesting methods of modifying it is medical advice. It's conceivably possible that you could leach out the plasticizer and make the bristles harder than the dentist would recommend, or that hydrogen peroxide could react with it to form some carcinogen, etc. Therefore I will say do not rely on any suggestions here for what you do with it. Wnt (talk) 01:51, 12 September 2012 (UTC)
- Humm, maybe you're right - though 'wash it with tapwater' is hardly medical advice I'd have thought... AndyTheGrump (talk) 01:54, 12 September 2012 (UTC)
- And in the best tradition of science reference desk original research, I can confirm that in some toothbrushes at least, a metal doo-dah is indeed used to retain the bristles. I've just sawn part of the head off one (used, needless to say - I'm not made of money ;-) ) and found a tiny sliver of metal, about 2mm x 1.2mm x 0.2mm (roughly - I've not got the tools to measure accurately) at the bottom of the brush-holes. AndyTheGrump (talk) 02:09, 12 September 2012 (UTC)
- This is all ridiculous. The mouth naturally contains a massive contingent of bacteria; a toothbrush can't possibly make a meaningful difference. Looie496 (talk) 02:19, 12 September 2012 (UTC)
- Obviously the toothbrush could make a difference. If you were living in Haiti and your toothbrush was five feet from the toilet somebody with cholera just used, you'd be reaching for the bleach! (Nay, come to think of it, in that situation who needs to brush...) Counting total numbers of bacteria is a common fallacy. Wnt (talk) 02:39, 12 September 2012 (UTC)
- Right, it's not just how many bacteria, it's which bacteria. Inside the mouth, the bacteria will normally be kept in check, but outside, you have a wet toothbrush with mouth bacteria, bits of food, and various toilet flushings sitting around like a petri dish. God only knows what will grow there. The ADA does suggest replacing brushes constantly, but I'm looking for a cheaper alternative. StuRat (talk) 03:40, 12 September 2012 (UTC)
- There appears to be a fair amount of dispute of whether you really need to replace your toothbrush due to bacterial contamination. There appears to be little dispute you probably should do it due to physical degradation. See [3] which while a forum discussion with a fair amount of typical forum nonsense does include some sourced information, include that the CDC say there is no evidence reusing toothbrushes can lead to (re)-infection. Nil Einne (talk) 08:15, 12 September 2012 (UTC)
- It's true that there are common soil microbes that can cause disease, like botulism or blastomycosis. But by and large, there is no evolutionary incentive for environmental bacteria to be harmful. So far as I know, most of the common food poisoning bacteria (like Salmonella or EPEC) come from humans or related organisms. And it seems hard to argue with the empirical observation that (unless someone ill is present in the environment) people don't generally seem to get sick from their toothbrushes, not even counting the demonstrable particulates from the toilet. It is possible to argue that theoretically there is some unnoticed rate of illness, but balance that against the hygiene hypothesis of asthma, i.e. that lack of exposure to microbes might be harmful. In the end we live in a world where there's a certain amount of turd in everything, whether it's from dust mites or the Old Faithful toilet. Wnt (talk) 15:14, 12 September 2012 (UTC)
- I don't think there could be a cheaper alternative to replacing toothbrushes constantly because dental care costs money too. The bristles also splay out after some use, making them less effective. But shaking out water after use and allowing to dry in circulating air seems like a good idea. There are two-half plastic holders for toothbrushes which have holes in them for air circulation. Sunlight would seem to be a good disinfectant but I haven't tried displaying my toothbrush on the windowsill yet. Apparently ultraviolet light is used for this purpose too. Vinegar as well as "3-percent hydrogen peroxide" are mentioned as possibilities here as well. Bus stop (talk) 15:37, 12 September 2012 (UTC)
- BTW I came across this earlier, it didn't seem that useful being a paid Colgate advert, but I now notice it mentions a study suggesting how much the effectiveness of a toothbrush can change over time [4]. Also looking at the ADA's current statement [5], even they don't seem to suggest contamination is a big reason for replacement, they too concentrate on physical degradation. (The replace due to contamination primarily comes in to play after you recover from certain diseases.) A number of suggested methods for cleaning a toothbrush are mentioned in the forum post I linked above including a dishwasher and mouth wash, but personally I'm with the person in this source [6]. Doing anything other then clean with room temperature water runs the risk you're going to damage the toothbrush more. (This includes sunlight/UV light.) And considering we have little real evidence of the risks of contamination, but evidence of the negative effects of physical degradation, there's a good chance you're causing more harm then you're preventing. Nil Einne (talk) 18:44, 12 September 2012 (UTC)
- Buy a new toothbrush? 203.112.82.128 (talk) 17:08, 12 September 2012 (UTC)
- Soak the bristles of the toothbrush in a small amount of mouthwash. — Preceding unsigned comment added by 148.177.1.210 (talk) 17:19, 12 September 2012 (UTC)
I should probably mention the reason I first used the bleach. My toothbrush smelled bad, and I didn't want that smelly thing in my mouth. To me, this was most likely the result of bacterial growth. StuRat (talk) 20:19, 12 September 2012 (UTC)
- Medical advice or not, it's safe to say that if your toothbrush smells bad, it's high time to get a new one. ←Baseball Bugs What's up, Doc? carrots→ 03:59, 13 September 2012 (UTC)
- Or stop cleaning your teeth, if you are really concerned that the apparently-minor risk of something nasty sneaking in on your toothbrush out-weighs the more obvious risks of not cleaning them - including quite possibly bad breath, which has the advantage that you are less likely to come into close contact with other germ-carrying individuals. Your choice... AndyTheGrump (talk) 04:26, 13 September 2012 (UTC)
- Did you read the referenced discussion above? It's not clear how disinfecting it is going to help the fact that by the time it starts to smell bad, it's likely sufficiently degraded to require replacement. If you only brush your teeth because someone will nag you otherwise, then I guess disinfecting it might help since there's a chance after several years there might actually be some risk from contamination. But if you're like most people and brush your teeth to actually clean your teeth and reduce the risk of dental disease, then the merits of disinfecting remain unclear. There's also BusStop's point. Perhaps you have gold plated dental insurance which you don't pay for or something, or you live in some odd corner of the US where toothbrushes cost a few hundred dollars. If not, the cost of a new toothbrush could easily be 2 orders magnitude less then a single visit to the density or extra surgery required. So the cost savings from not replacing your toothbrush every few months are fairly unclear as avoiding even a single extra surgery or visit in 10 years could easily pay for replacing your toothbrush every 2 months in the same timeframe. Nil Einne (talk) 04:36, 13 September 2012 (UTC)
- Where I live, I can get toothbrushes 4 for a dollar at the local dollar store. Certainly, I can pay more, but with the cheapest toothbrushes I can buy, I could replace once a month at a cost of less than a penny per day. At that level, the cost of replacing a stinky toothbrush shouldn't be an issue. --Jayron32 05:02, 13 September 2012 (UTC)
- If the toothbrush becomes rancid quickly, the problem might not be the toothbrush. It's time for StuRat to pay a visit to his dentist. ←Baseball Bugs What's up, Doc? carrots→ 11:26, 13 September 2012 (UTC)
Strange new observation: I dumped out the bleach and replaced it with mouthwash. The next day, the mouthwash had changed from green to blue. Could it have reacted with some remaining bleach ? StuRat (talk) 06:51, 15 September 2012 (UTC)
- Sounds likely. Bleach is highly alkaline[7], mouthwash is slightly acidic, and some of the color additives of the mouthwash may have pH indicator properties (although I could not identify any such substances, after googling the color additives of various brands of mouthwash). Another possibility: bleach is known for degrading colored substances. The green color have been produced by a mixture of blue and yellow dyes. Maybe the bleach degraded the yellow dye, but not the blue one. --NorwegianBlue talk 21:17, 15 September 2012 (UTC)
Global Ischemia and Death[edit]
I'm wondering about this--are people with global ischemia (who get revived) completely dead for several minutes or just don't have a functioning brain (brain activity) for those several minutes while the rest of their body works properly? Also, people with global ischemia can have their whole "mind" (personality, likes, hobbies, etc.) restored if they had global ischemia for several minutes or less, right? Futurist110 (talk) 02:18, 12 September 2012 (UTC)
- It helps if you provide a link to what you are talking about. μηδείς (talk) 02:34, 12 September 2012 (UTC)
- (ec)Hmmm... brain ischemia#global brain ischemia speaks of restoration after loss of heartbeat. Clearly neurons don't die the instant blood stops pumping - it takes time for the oxygen to run out, among other problems. The death is not a physical law - it can be altered by methods such as treatment with sodium valproate. [8] Deciding when a cell is dead is an example of something that is very, very difficult to define philosophically but all too easy to measure by experiment... Wnt (talk) 02:36, 12 September 2012 (UTC)
- If they come back, their brains did not entirely cease to function. If electrical activity in the brain completely disappears (sometimes known as "flatlining"), the patient is dead, dead, dead, never to return, end of story, dead. Looie496 (talk) 05:29, 12 September 2012 (UTC)
- Actually our article on brain death gives the impression that this is not absolutely certain, though with the caveat that the activity might simply not be detectable with the equipment used. It's unsourced there - ought to come up with better references... Anyway, in theory there's no reason why neurons couldn't stop firing entirely for some reason (a drug that blocks the action potential) and yet, if life support can be maintained, return later to normal function. Wnt (talk) 15:35, 12 September 2012 (UTC)
I was under the impression that electrical activity in the brain stopped completely with global ischemia? Am I wrong? Futurist110 (talk) 23:04, 12 September 2012 (UTC)
Late summer sky color[edit]
I have often noticed that in the Eastern and Midwest U.S., the sky takes on a hauntingly beautiful shade of blue in the late summer and early fall (this year, starting August 13). The sun takes on a similarly more attractive shade of golden, and together, the impact of the blue and golden on the dark vegetation of summer can be just breathtaking. At night, especially later in the season, the sky can take on a uniquely "electric" indigo shade. What's odd is that it seems so specific to this season, yet it can occur in 90-degree weather or 50-degree weather, in cloudless sky, sky half filled with cumulus clouds, sky marked with the strange swirls of cirrus that came with this week's unusually cold weather. I still do not even know for certain that it is not some purely psychological phenomenon on my part. Is this effect something known? And if so, is it explainable? Wnt (talk) 02:55, 12 September 2012 (UTC)
- I don't have a specific answer, but in general the specific color of the sky is highly dependent on the exact angle which the sun's rays are striking the atmosphere, and this angle will be highly dependent on both the time of day and the time of the year. The coloration of the sky is largely due to processes called Rayleigh scattering and Diffuse sky radiation, and accounts for the blue sky at midday and also the red sky which appears just before sunset. So, I don't know exactly what combination of effects causes the specific shade of blue you are noting (though your vivid description brought it to my mind as well!), except to direct you to the general processes that create the sky's color; it is likely just a specific combination of the normal variables that affect the color of the sky in general. --Jayron32 03:47, 12 September 2012 (UTC)
- Just a thought: Do you happen to go out at the same time each night, for a walk, say ? If so, then this might be the time of year when your walk time corresponds with sunset. There should be a second time in spring, but maybe it tends to be rainier then. StuRat (talk) 04:03, 12 September 2012 (UTC)
- Particulates high in the atmosphere can cause pretty sunsets. These are caused by volcanoes, forest fires, and perhaps sandstorms. Volcanoes shouldn't vary with the weather, but fires might, and sandstorms in Africa do sometimes carry particles over to the US. StuRat (talk) 04:22, 12 September 2012 (UTC)
- The sky I saw tonight went from a brilliant cyan with magenta clouds just before sunset to a rich emerald and then a deep teal after. I don't think there's anything like a volcanic eruption going on in NJ. I though particulates usually cause red sunsets. Is there some current eruption or fire in the Northern Hemisphere that has been reported as causing brilliant sunsets? My suspicion is what I saw was more due to the clarity of the sky and the angle of the sun, but I have never seen it greener. I also remember the sky on 9/11 being one of the clearest and most brilliant I have ever seen. μηδείς (talk) 05:00, 12 September 2012 (UTC)
"Strike as non-responsive."
|
|---|
|
- It's fair humor - I flew out of Newark a couple of times in the 80s and the city deserved the gibes then; maybe they helped prod it to clean up its act (which as I understand, it has done significantly) and if so, by virtue of saving many human lives, these jokes' ethics are beyond reproach. And for our question, artificial sources of particulates certainly should be considered seriously. That said... I'm not sure any industrial pollutant would be worse this particular time of year, though the great decrease in rainfall should reduce the amount that is purged from the atmosphere. My gut feeling, of course, is that pollution couldn't possibly be so pretty, but that's not a scientific argument. :) Wnt (talk) 14:54, 12 September 2012 (UTC)
- I think of this as a regular yearly phenomenon (subjectively to me, this effect together with the singing of cicadas and katydids define a season of "latesummer"). So I would discard thoughts of an eruption out of hand. Sahara dust is my leading candidate, but quick searching points to it creating haziness in Florida [9] and being limited to the South [10]. Even so, I suppose that a super fine subpopulation of the dust might make it further north and have these effects? But without seeing proof, I don't really know. Wnt (talk) 05:16, 12 September 2012 (UTC)
- Medeis: do you mean the Green flash? Ssscienccce (talk) 16:15, 12 September 2012 (UTC)
- No, I couldn't actually see the sun itself when it set, the entire sky was a distinctly greenish shade near sunset, the likes of which I have seen on maybe two or thee other occasions, somewhat like the teal green in our article, but luminous, of course. And I am not sure how true the colors are on my computer, so don't 'quote' me on that. It will be interesting to see if the weather is the same today. μηδείς (talk) 17:16, 12 September 2012 (UTC)
- Medeis: do you mean the Green flash? Ssscienccce (talk) 16:15, 12 September 2012 (UTC)
The sky was very similar again last night, with green, orange and grape-soda purple at the horizon about an hour before sunset, depending on which direction you were looking in, although I was too busy to see the color overhead at sunset. Not a single cloud was visible, which makes me think the humidity and sun angle are relevant. μηδείς (talk) 17:53, 13 September 2012 (UTC)
Janet Armstrong[edit]
What happened to the first wife of Neil Armstrong after her divorce? I've looked on the internet but she's not been mentioned except in one New York Times article about events in Neil's life where it briefly mentions that she lives in Utah. I'm wondering if she ever re-married and when she ended up moving. If this is in the wrong place, please just move it to the correct area and write where you put it on my talk page; I wasn't sure if this counted as humanities (because it was biographically related to Neil Armstrong's life) or here (because Neil Armstrong was the first man on the moon) so I chose here. --Thebirdlover (talk) 03:30, 12 September 2012 (UTC)
- Doing a people search on Janet Armstrong and her maiden name, Janet Shearon, I only found one that seems old enough, a 75 year old Janet Ann Armstrong, who has lived in Pahrump, Henderson, and Las Vegas, NV, as well as Apache Junction, AZ and Stockton, MO. No guarantee this is her, but it's possible. Does anyone know if her middle name was Ann ? StuRat (talk) 03:59, 12 September 2012 (UTC)
- Not the right one. His first wife was Janet Elizabeth Shearon, you might need to scroll down a bit. CambridgeBayWeather (talk) 23:58, 12 September 2012 (UTC)
Use of question mark and exclamation mark above equals signs[edit]
I have seen the notation and used before, and I have forgotten where. (It may have been in a highschool math textbook, maybe)
: "we would like to show that X equals Y", or "we wonder if X eqals Y"
: "Yes, X does indeed equal Y, as is now obvious"
These notations are not entirely unknown on the internet, I have found several stack overflow threads in which people ask how to produce them in LaTeX, and other threads in which people ask what they mean. However, it appears they are less well-known than I thought. So, I am looking for actual mathematics/physics papers in which these symbols are used. 81.11.174.45 (talk) 06:19, 12 September 2012 (UTC)
- I think that must be very well-known. I use it in my scratch notes when I'm trying to prove something. However, a search on Wikipedia fails to find any articles here that use it. But I'm sure it's out there somewhere.
- As for , I don't recall seeing that before, though its meaning seems obvious. Duoduoduo (talk) 21:22, 12 September 2012 (UTC)
- My feeling is that they are primarily used in notes, blackboards, and textbooks; not research papers. Really, starting with the first symbol and terminating with the second is like starting with an ansatz and ending with a Q.E.D.. My point being, researchers are likely to handle these issues with words rather than special symbols whose meanings would have to be carefully defined. SemanticMantis (talk) 21:30, 12 September 2012 (UTC)
- This German language mathchat site uses it. I don't read German so I don't know if it might help you find some published papers on the chat topic that use it. Also this one. Duoduoduo (talk) 21:46, 12 September 2012 (UTC)
- It's hard to search for this, but coincidentally a few hours after seeing your question I spotted in Exploratory Experimentation and Computation by Bailey and Borwein. I think I've never seen , and I would probably guess that it meant "is, surprisingly, equal to", not "has now been shown to equal". -- BenRG (talk) 00:50, 13 September 2012 (UTC)
- Can I just say in passing what a joy it is to learn new things on the reference desks. Had I not seen this thread, I could only have interpreted as an enquiry as to whether I have a predilection for liquorice allsorts. Now I know. AndyTheGrump (talk) 04:07, 13 September 2012 (UTC)
Gravitational pull vs. push[edit]
I saw a documentary about exoplanets the other day. They were talking about the way exoplanets make their stars wobble slightly, and that that wobbling can be detected by telescopes, and thus the exoplanets can be detected although they don't reflect enough light to show up themselves.
One of the animations was way off, though. The planet was moving around the star in a circle, and the star was moving more slowly. But the star was always moving in the same direction. Ascii art:
* .
. *
* .
- etc.
As far as I can tell, that kind of orbit means that the planet is attracted by the star, but actually repelling it. I think of this motion that it's plain impossible, however I tried to look at the math and find a solution to prove that it's impossible. My best result so far was that, to make that motion plausible, the center of gravity must stay at the same place during the orbit, or move at a constant speed and in a constant direction. If I call the masses involved M and m, and the locations of the bodies X and x, I get an equation like
- M X(t) + m x(t) = c, c being independent of t and of dimension (kg m).
thus if both locations have the same sign, the m asses must have opposite signs, not exactly a common property among celestial bodies.
Which would result in m repelling M, but also in M repelling m.
So, while the negative masses seem to prove the animation wrong, I proved at the same time that there is no stable orbit at all. As sure as I am that the animation is wrong, my calculations look wrong too, but I cannot seem to nail the error.
Is it in assuming M and m independent of t? They should be if they were moving in perfect circles? - ¡Ouch! (hurt me / more pain) 07:54, 12 September 2012 (UTC)
- In a 2-body system, both objects should orbit about the barycenter (center of mass) of the system. The barycenter will be much closer to the star, perhaps even inside it, but not at it's center. So, yes, the animation looks off (was this the Discover Channel, by any chance ?). In your equation, it isn't one of the masses which is negative, but rather one of the displacements, where negative means it is in the opposite direction from the barycenter as the other displacement. StuRat (talk) 09:49, 12 September 2012 (UTC)
I know that X(t) and x(t) are the pair which have opposite signs in real life. The point was that I wanted to explore what configuration could exhibit the behavior the animation showed, i.e. the data they fed into the computer in the first place, to produce such a degenerate case of orbit. A sign error looks quite possible, as the bloody comp will process any parameters you feed it no matter how whack-off they are.
If M and m have opposite signs, the barycenter is no longer between the two bodies, though. Effectively, if we put the barycenter into the origin, the equation simplifies to
- M X(t) = - m x(t),
which does not explain either what repels the star from the planet.
Sorry, can't say if it was on DC, I was so waisted it could have been on Complete Nonsense Network and I wouldn't remember. Now I'm sober and my brain is still doing barrel rolls trying to find the parameters for that kind of orbit. - ¡Ouch! (hurt me / more pain) 10:51, 12 September 2012 (UTC)
- Looking at the real world case, if we start with your equation:
M X(t) + m x(t) = c
- But let's just use 0 for the constant:
M X(t) + m x(t) = 0
- Now make one of the displacements negative:
M X(t) + m (-x(t)) = 0
- We get:
M X(t) - m x(t) = c
- Moving it to the other side, we get:
M X(t) = m x(t)
- If you're trying to figure out an equation to produce that nonsense animation, that seems like a pointless exercise. I suspect they just entered circular orbits for both into their animation software, around a point not between the two. So, they defined two independent circular orbits, not taking gravitational attraction into account at all. Maybe you can imagine both orbiting about a black hole, to make it all make sense (although the orbital speeds would be different, in this case). They probably weren't using astronomy software at all, but just some general animation program, which is happy to let you define circular motion however you want, with no consideration made for gravity. StuRat (talk) 11:05, 12 September 2012 (UTC)
I think I found the answer myself. Classifying forces as "attracting" and "repelling" is misleading if masses can be negative. Newton's F = m a shows that acceleration changes sign if the mass does. What this means is that a planet will orbit a star in the same way regardless of its mass (assuming it is much less than the star).
Say, the planet is Earth, m = mE.
Then, gravity is F = G M mE / (X(t) - x(t))2, and a = G M / (X(t) - x(t))2.
If the planet's mass is v times the mass of Earth, we get
F = G M v mE / (X(t) - x(t))2, and a = G M / (X(t) - x(t))2.
v cancels out, including the case v < 0. Negative inertia is a bit counterintuitive.
However, the impact the planet has on the star does depend on m. And the original solution was correct. I was wrong in thinking that there wouldn't be a stable orbit. The negative inertia fooled me.
The more important point is, however, (as you pointed out, Sturat) that they probly didn't feed any simulation data into the animation, and that their "scientific advisors" were too dazed (even more so than I was???) to catch their mistake.
And thanks for not picking up on "waisted", ;) - ¡Ouch! (hurt me / more pain) 07:01, 13 September 2012 (UTC)
/ \/ Resolved. </barrel roll>
- You're welcome. I'm amazed at how fake many documentaries and science shows are. I once saw one featuring Hero's engine which showed it rotating the wrong way. I can only conclude that they rigged an electric motor to it, to get it to spin. Then I saw a show where they demonstrated "the proper way to build a campfire". They insisted the large logs go on the bottom, then medium sized on top of those, then small twigs, with kindling on the very top. They said this is needed to keep it all stable. Only problem, is, of course, you could never light a fire this way. They were having no luck, until they went to commercial and came back, and it was blazing away like it had just been doused in gasoline. :-)
- And here's a proper resolved tag, although yours was a valiant effort. :-) StuRat (talk) 07:20, 13 September 2012 (UTC)
sound to electrical energy conversion[edit]
sound is a form of energy. each day we speak a lot i.e we waste a lot of energy. i want to use this energy. as well as there is a lot of noise in surrounding. how can we utilize this? atleast we can charge our cell phone. so, how can i convert this energy into electrical energy? — Preceding unsigned comment added by 110.172.159.134 (talk) 12:44, 12 September 2012 (UTC)
- Sound isn't very much energy. The Io of Sound intensity level is 10-12 w/m2. (Compare with the roughly one kilowatt per meter squared of sunlight. I.e. if we could "hear" sunlight it would be at 150 dB, which is well above the threshold of pain. And I don't see very many directly solar powered cars on the road. (Instead they use liquified fossilized sunshine.)) Hcobb (talk) 14:09, 14 September 2012 (UTC)
- To elaborate on that, according to the source our article cites at [11], the sound intensity level of 60 dB SIL "conversational speech" is 0.000 001 W/m2 Assuming you're in a big cubical room of, oh, 10 meters on a side, a speaker being heard anywhere in the room would need to achieve this over 600 square meters, which would take 0.0006 watts of power. Now note that someone with a basal metabolic rate of 1500 kcal/day = 6300 joules/day = 0.08 joules/second (watts) is expending 1300 times more energy lying in bed than is actually needed for you to hear him in conversation, which tends to sap the drive for improved efficiency. (Hope I didn't foul up the math again...) It would be interesting to make the same calculation for a cicada which puts a higher priority on being heard. Wnt (talk) 15:27, 12 September 2012 (UTC)
- There are instruments that convert sound to electrical energy. They are called microphones and your cell phone has one. --Wrongfilter (talk) 15:44, 12 September 2012 (UTC)
- The only practical use I know of is a sound-powered telephone using a balanced armature design for efficiency. Ssscienccce (talk) 16:11, 12 September 2012 (UTC)
- A seismograph also uses the energy in vibrations, including sound, to drive the needle. StuRat (talk) 20:12, 12 September 2012 (UTC)
Laser Light as Propulsion?[edit]
Would it be possible, theoretically, to use the 'pressure' of laser light to move a solar sail through space? Or would Newton's Third Law, (i.e., "every action produces an equal and opposite reaction") prevent this?Honeyman2010 (talk) 17:37, 12 September 2012 (UTC)
- We have a Laser propulsion article, which talks mostly about ground-based lasers hitting spacebourne vehicles. -- Finlay McWalterჷTalk 17:40, 12 September 2012 (UTC)
- In terms of the physics of photons and momentum, there's no reason the laser can't be on the craft. "Couldn't" only enters in once you're talking about engineering tradeoffs. — Lomn 18:19, 12 September 2012 (UTC)
- I may have been unclear. If the laser is on the craft and you fire it at a sail attached to the craft, you aren't accomplishing anything. You could however fire the laser into empty space, using it as a sort of rocket. That would be a terrible strategy, though, since you would be using a maximum of energy to get a minimum of momentum. Looie496 (talk) 18:56, 12 September 2012 (UTC)
- With current technology, that's quite true. However, if you had a nearly infinite source of energy, let's say we get on-board fusion reactors working, and didn't need much thrust, let's just say "station keeping" (maintaining an orbit), then it might be more of a reasonable choice to launch particles at the speed of light. However, there's no need for the particles to be collimated, unless you need the beam to stay together to also drive another vehicle (say when two spacecraft separate). Since very little mass is ejected when you launch particles at the speed of light, you don't need to be worried about running out of material. StuRat (talk) 20:08, 12 September 2012 (UTC)
- Regarding a laser-firing-at-its-own-craft's-sail—how (and how well) it works depends on what the sail is made from. If it is a reflective material, then the sail will reflect the beam and give you the same amount of thrust as pointing the beam straight into space, albeit in a different direction. If the sail is nonreflective, then it will absorb the laser light and heat up; eventually it will emit blackbody radiation from its face and produce thrust in that way. It only fails to produce thrust if all of the reflected/emitted light is recaptured by other parts of the spacecraft, or if the sail is both nonreflective and sufficiently thin and thermally conductive that blackbody radiation is emitted equally from both of its faces.
- I can see the potential value of a small reflective sail on a photon rocket; the sail would be used to vector thrust without requiring reorientation of the entire laser. TenOfAllTrades(talk) 21:47, 12 September 2012 (UTC)
- Lasers are used because they allow a distant sail projectile to be targeted. If you don't need to do this, and you're carrying the light source on-board the vessel, then you don't need to use a laser and you don't need to suffer the laser's inefficiencies. You can use a simpler and more efficient light source. Andy Dingley (talk) 20:28, 12 September 2012 (UTC)
- Lasers of sufficient strength are big and heavy and require big and heavy power sources. They also don't let out a huge amount of energy for all of that weight and trouble. Their one good thing is that they can transmit their energy over long distances. So this is why most laser propulsion schemes are about putting the big, heavy, energy-hungry part — the laser — on the ground, and using it to project a high-energy beam of light to something that is lightweight, pushing it along. If you aren't going to do that, then you don't get anything beneficial out of laser propulsion. --Mr.98 (talk) 18:50, 12 September 2012 (UTC)
See also problem 7.5 on page 13 of this set of test problems. Count Iblis (talk) 19:48, 12 September 2012 (UTC)
Also, we have the article photon rocket. Count Iblis (talk) 19:54, 12 September 2012 (UTC)
- Also, the Pioneer 10 and 11 spacecraft served as an unintentional tech demonstration of this effect. See: Pioneer anomaly. Though the photon source was a 150W RTG, and the thrust produced "not very much". 8.74±1.33×10−10 m/s2 or so. After 40 years running its "photon engine", its now about 1mph slower (because the RTG is pointing outward- in the direction its moving).--Robert Keiden (talk) 00:28, 13 September 2012 (UTC)
- Exponents repaired for clarity. - ouch
- Laser beams can ping-pong between two mirrors to get more propulsion out of them, too (OR). The beam will red-shift if the moving mirror is moving away or blue-shift if it's moving towards the fixed mirror.
- Fusion is not even close to a near-infinite power source BTW. It would be of greater benefit to tap the magnetic bottle for a burst of high-v particles, rather than tapping that kinetic energy to power a light source. The average v of fusion particles is around 0.12c = 36000km/s.
- Kinetic energy of 1kg of photons: E = m c² = 9x1016Ws = 2.5x1010kWh,
- Momentum of 1kg of photons: m c = 3x108kgm/s.
- Kinetic energy of 1kg of fusion particles: E = (m/2) v² = 6.5x1014Ws = 1.8x108kWh,
- Momentum of 1kg of fusion particles: m v = 3.6x107kgm/s.
- That is, using the kinetic energy of 1kg of fusion particles, you can emit ~7g of light for a momentum of ~2x106kgm/s, or use the particles directly for roughly 18 times that momentum. The savings will only be offset if the power source is fixed (which is feasible only with a laser since you cannot project a focused particle beam into space).
- OtoH, the Pioneer anomaly does look like a workable photon propulsion, and it doesn't need a full-scale reactor core either. Once you escape Earth's gravity, it's literally smooth sailing. A parabolic mirror, a lump of radioisotopes at its focus, and a set of attitude control thrusters, and there you go, slowly spiraling outward.
- p.s. If the mirror reflects (or merely catches) neutrons, it's even better, because their momentum-to-energy ratio is superior to photons, either. - ¡Ouch! (hurt me / more pain) 07:57, 13 September 2012 (UTC)
- Interesting calcs, but what are your assumptions ? Are we talking about fusing normal hydrogen into normal helium ? How about if the reactor continues until you get iron ? You should be able to get more energy out of fusion that way. Also, you can eject the reaction products at high speed, too.
- If you leave the laser here on earth (or preferably, on the moon or in orbit someplace), there is even a way to have the spacecraft decelerate and eventually fly back towards the laser for the 'return trip'. Have the craft carry two sails - deploy one in the conventional manner on the outward journey - then when you need to decelerate, you cut the lines holding it and deploy a second sail pointing in the opposite direction - laser light reflects off of the detached sail (which continues to accelerate away) and onto the newly deployed sail and the craft will be able to decelerate, do whatever needs to be done - and eventually return home. The real problem is the power of the laser required to do this. Even if it's left back here on earth, keeping the beam sufficiently well aimed and focussed that enough of it's light is 'caught' by the sail is a tough problem that gets worse and worse the further the craft gets - the increasing time delay for the craft to report back with laser realignment instructions would also get seriously problematic as distances increase. SteveBaker (talk) 13:29, 14 September 2012 (UTC)
Seeing Our Own Creation[edit]
Telescopes are making huge leaps in their ability to look further and further back in time, and to see greater detail than ever before. I believe astronomers have even captured images of protostars and proto solar systems, and imaging actual new planets is not too far in the future. Therefore, since we can see the creation of other solar systems, isn't it accurate to say that every event since the creation of our solar system is currently being carried by photons created at the time and now a maximum of 4.7 billion light years away from us? The creation of the moon, the extinction of the dinosaurs, etc., all luminous events could be entering some distant civilization's telescopes at this moment.
If this is the case, and if there is really no 'center' to the universe but rather we are all on this ever expanding balloon of space-time, is there any scenario where we could even capture those photons which would show us our own creation?Honeyman2010 (talk) 22:44, 12 September 2012 (UTC)
- It would seem unlikely. They're speeding away from us at the speed of light. We'd have to go faster than the speed of light to be able to interact with them so as to gather their information payload. Faster than light is, I think, forbidden in this universe. --Tagishsimon (talk) 22:49, 12 September 2012 (UTC)
- We could certainly never catch up with those photons, but they may be returned to us by a couple of means. Theoretically, a very strong gravitational lens might return light from the early solar system back to us, although it may be dim and distorted beyond all recognition. Furthermore, if the universe is finite in extent but sill centerless (as in a [hyper]spherical universe), that light will eventually return to us regardless, but that may be a very long time in the future, which would mean it's very dim, and we'd need a freakin' huge telescope to view it. Someguy1221 (talk) 22:51, 12 September 2012 (UTC)
- We've detected extrasolar planets up to 50,000 light years away, which is an impressive half of our galaxy away. In contrast, Andromeda galaxy is one of the closest galaxies to us and it is 2.5 MILLION light years away. You are asking about planetary sized objects 4.5 BILLION light years away? I don't know if I'd go so far as to say it's "impossible", but I won't be holding my breath.. Vespine (talk) 23:36, 12 September 2012 (UTC)

- At 4.5 Gly, the Milky Way, Andromeda and Triangulum galaxies would all blur together. This is what a cluster of 100 galaxies looks like, from about the same distance: --Robert Keiden (talk) 00:56, 13 September 2012 (UTC)
- It's really just a technical limitation. A sufficiently large telescope could view the pimples on your face from 4.5Gly away, although that telescope may itself be light years across. I can't remember how to calculate the limits. Someguy1221 (talk) 01:23, 13 September 2012 (UTC)
- No, it is more than a technical limitation, it is a physical limitation as well. In order to view your pimple, the telescope would have to capture some number of photons from that pimple, and at certain distances, you just can't capture enough photons. See Shot noise for a discussion of some of the problems. --Jayron32 01:31, 13 September 2012 (UTC)
- Nevertheless, Hubble Ultra-Deep Field managing to image objects as they were ~13 billion years ago is pretty cool. Sean.hoyland - talk 03:40, 13 September 2012 (UTC)
- Sure, but the objects it is seeing are considerably larger than the pimples on my face. --Jayron32 03:43, 13 September 2012 (UTC)
- So here's a factual question, assuming we have unlimited resources to build arrays of interfering telescopes, at what distance do certain things become unobservable? Individual stars, Earth-like planets, cities, people on those planets, etc. My claim came from the recollection of an astronomy lecture years ago that discussed the theoretical construction of a telescope array that could resolve to one pixel a person's head on a planet 50 light years away. It hadn't occurred to me that noise would put a limit on how far something could be seen even with an arbitrary telescope. So, back to my question, assuming a sun-like star and an Earth-like planet in orbit, typical interference from intergalactic/interstellar gas, at what distance does noise preclude any observation? Someguy1221 (talk) 06:58, 13 September 2012 (UTC)
- There's possibly no limit to the math involved, I suppose, but you start to get rediculous when it comes to actually constructing a telescope. For example, if you build a lens so large that it conatains all the mass of the known universe, or you build one so large that light could not travel from one end of the lens to the other fast enough to get there before the heat death of the universe, or the telescope is so large that its own mass causes it to collapse into a black hole, or any of a number of other ludicrous propositions, well, I don't think we're dealing with mere "technical limitations" anymore. That is, if you narrow yourself to just those equations that relate the size of a lens to its resolution, you could build a telescope of any size and have nearly infinite resolution. But that is only if you ignore all of the other laws of physics that come into play, and if you're going to ignore some set of the laws of physics, you're doing no better than invoking "magic". The article, by the way, that will answer the main question about how "far" we can see is Optical resolution, and sure, using just those equations, you could construct a telescope of any size which could resolve to any arbitrary accuracy at any arbitrary distance. But there are other considerations when constructing such a telescope, and there are limits to the size a telescope can get before other laws of physics start to make infinite resolution at any distance impossible. --Jayron32 13:10, 13 September 2012 (UTC)
- So here's a factual question, assuming we have unlimited resources to build arrays of interfering telescopes, at what distance do certain things become unobservable? Individual stars, Earth-like planets, cities, people on those planets, etc. My claim came from the recollection of an astronomy lecture years ago that discussed the theoretical construction of a telescope array that could resolve to one pixel a person's head on a planet 50 light years away. It hadn't occurred to me that noise would put a limit on how far something could be seen even with an arbitrary telescope. So, back to my question, assuming a sun-like star and an Earth-like planet in orbit, typical interference from intergalactic/interstellar gas, at what distance does noise preclude any observation? Someguy1221 (talk) 06:58, 13 September 2012 (UTC)
- Sure, but the objects it is seeing are considerably larger than the pimples on my face. --Jayron32 03:43, 13 September 2012 (UTC)
- Nevertheless, Hubble Ultra-Deep Field managing to image objects as they were ~13 billion years ago is pretty cool. Sean.hoyland - talk 03:40, 13 September 2012 (UTC)
- No, it is more than a technical limitation, it is a physical limitation as well. In order to view your pimple, the telescope would have to capture some number of photons from that pimple, and at certain distances, you just can't capture enough photons. See Shot noise for a discussion of some of the problems. --Jayron32 01:31, 13 September 2012 (UTC)
- It's really just a technical limitation. A sufficiently large telescope could view the pimples on your face from 4.5Gly away, although that telescope may itself be light years across. I can't remember how to calculate the limits. Someguy1221 (talk) 01:23, 13 September 2012 (UTC)
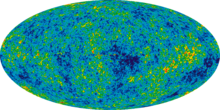
- This question has a rather unambiguous answer. The furthest back in time we can see is the cosmic microwave background radiation, before that the universe was "opaque"; i.e., a glowing cloud we can't see through. Here is an image. μηδείς (talk) 17:47, 13 September 2012 (UTC)




