Wikipedia:Reference desk/Archives/Science/2010 July 8
| Science desk | ||
|---|---|---|
| < July 7 | << Jun | July | Aug >> | July 9 > |
| Welcome to the Wikipedia Science Reference Desk Archives |
|---|
| The page you are currently viewing is an archive page. While you can leave answers for any questions shown below, please ask new questions on one of the current reference desk pages. |
July 8[edit]
WTF is that on your ear? AKA fleshy protuberance on the targus?[edit]
Here in China I often see people walking around with - for lack of a better word - ``growths`` just in front of their ears - basically just ahead of the targus. Sometimes they're quite small, perhaps 2~3mm tall. Other times they can exceed 1cm in length. Never in my life have I seen these outside of China. I suspect that's because other cultures remove them for cosmetic reasons? In any case, I am VERY curious what causes these growths? what they're called? etc... 218.25.32.210 (talk) 05:21, 8 July 2010 (UTC)
I think the word is tragus 86.4.183.90 (talk) 07:01, 8 July 2010 (UTC)
- Is it a Preauricular skin tag? I've noticed them in China, too. Maybe it's more common to remove them at birth in the west? —Preceding unsigned comment added by 128.12.174.253 (talk) 06:10, 8 July 2010 (UTC)
I have heard that Lego blocks can be used to make even functionable robots that's with motors and all, how i's done ? Jon Ascton (talk) 10:42, 8 July 2010 (UTC)
- You would need additional parts such as motors and bearings and hinges. --Chemicalinterest (talk) 11:17, 8 July 2010 (UTC)
- By using the Lego Technics ®/ Lego Mindstorms® kits. CS Miller (talk) 11:37, 8 July 2010 (UTC)
- Yes! Lego Mindstorms sets make that really simple. You get motors, gears, wheels, a small computer module, some switches and (in some sets) rotation sensors and light sensors. The computer can be programmed remotely from your PC using either a C-like programming language or in a 'drag and drop' environment where you build things that are like flow charts. People have built some rather impressive robots and both NASA and the "FIRST Lego League" run competitions of various kinds for Lego robots. There is a thriving user community of 'AFOLs' (Adult Fans Of Lego) - of which I confess to being one - they too have occasional challenges (things like: "Build a robot to stack empty coke cans - the biggest stack wins - send in a video of your robot stacking cans - the tallest stack wins"). The Mindstorms sets cost a couple of hundred dollars - and contain enough to do some reasonably complex projects - but it definitely helps to have a larger stock of more traditional Lego and Lego Technics to give you a wider range of component choices. There is also a pretty good used market for such things when parents belatedly discover that their 'little darling' isn't a gifted with computers as they thought! SteveBaker (talk) 18:30, 8 July 2010 (UTC)
- Are there any YouTube videos available of these impressive feats please? 92.24.188.89 (talk) 18:49, 8 July 2010 (UTC)
- Searching http://www.google.co.uk/search?q=Lego%20Mindstorms&um=1&ie=UTF-8&tbo=u&tbs=vid:1&source=og&sa=N&hl=en&tab=wv seems to work.
- This seems suitably AWESOME!!! http://www.youtube.com/watch?v=eaRcWB3jwMo (got a bit carried away).87.102.42.55 (talk) 20:00, 8 July 2010 (UTC)
- Are there any YouTube videos available of these impressive feats please? 92.24.188.89 (talk) 18:49, 8 July 2010 (UTC)
KBr (Potassium Bromide)[edit]
Potassium Bromide was called in medical education?--אנונימי גבר (talk) 13:28, 8 July 2010 (UTC)
- Do you mean Kalium bromide or Kalii bromidum [1] aka Kalium bromatum or Bromide of Potash ? 87.102.42.55 (talk) 13:56, 8 July 2010 (UTC)
- Are you asking "What was it called in medical education?". --Chemicalinterest (talk) 15:22, 8 July 2010 (UTC)
"Shadow Biosphere" life on earth with arsenic DNA backbone?[edit]
Last night on the program "Through the Wormhole with Morgan Freeman," this researcher found some cells in Mono Lake that could survive in an environment with arsenic levels thousands of times higher than what most life could stand. The woman sheepishly (to my ears) said it's possible the DNA backbone of the cells in question have arsenic in place of phosphorus, but she just left it at that. Isn't there a way to verify that? (P.S. I read the timesonline article sourced in the Mono Lake article about this exact scientist of whom I am speaking. I'm just surprised it doesn't seem they can verify the composition of a DNA molecule there in their petri dish. I thought we could do that these days.) 20.137.18.50 (talk) 13:53, 8 July 2010 (UTC)
- from search of "DNA Arsenate" see [2] quote
The only stumbling block to the idea is that arsenic-based DNA tends to break down quickly. "You don't want to build your DNA out of a compound with a half-life in the order of a couple of minutes," points out Steve Benner of the Foundation For Applied Molecular Evolution in Gainesville, Florida
- Yes arsenic can be detected accurately in molecules - but with only a few percent substitution it could be difficult.87.102.42.55 (talk) 14:06, 8 July 2010 (UTC)
is it present? The skeletal structure looks funny .... does it reflect real life? John Riemann Soong (talk) 15:44, 8 July 2010 (UTC)


- The Haworth projection grossly exaggerates the angular difference between 'equatorial' and 'axial' bonds on the sugar structure.
- The ball and stick picture is more accurate - the 6ring is standard (chair) formation, the 5 ring is standard formation too (envolope)
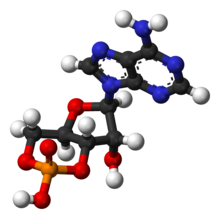
more accurate 3d image - If the question was about the ball and stick image - I think the angle chosen and blue heterocyclic structure makes the image look slighly odd- but it's not - note the two sets of rings are at right angles to each other - so the image has been projected at a slightly 'non-aesthetic' angle.87.102.42.55 (talk) 16:41, 8 July 2010 (UTC)
stimulation of exocytosis of nanoparticles by administration of 8-Br-cAMP[edit]
8-Br-cAMP is a lipophilic source of intracellular cAMP that can be added to solution. If I added some to epithelial cells (HeLa, lung cancer, etc.), should I expect gold particles trapped in vesicles to be exocytosed faster? Or would it do nothing if they hadn't reached the ER or a lysosome yet? John Riemann Soong (talk) 16:03, 8 July 2010 (UTC)
I have a point-and-shoot digital camera[edit]
It came with panasonic alkaline batteries. They used up in a day or two. OK. Then I changed them, and put brand new zinc chloride batteries, it shows "battery exhausted" even though they are brand new. Why ? Or should one only use alkaline batteries ? What's wrong ? Jon Ascton (talk) 16:17, 8 July 2010 (UTC)
- Take an ohmmeter across the terminals in your camera. Is the resistance unusually low? 20.137.18.50 (talk) 16:38, 8 July 2010 (UTC)
- Some ohmmeters use a high enough voltage that they would damage some electronic devices. I would instead measure the current drawn by the device from the usual battery, and perhaps the voltage the battery is putting out under the same circumstances. Care is needed to make sure the battery polarity is correct, the meter is connected with the correct polarity if an analog one, and the battery is not getting shorted out when such a test is done. Sometimes I have made a current probe by inserting as an insulator a piece of paper or very thin plastic between the battery terminal and the battery contact, or between two batteries, with a thin piece of metal on either side of the insulator, which get connected to the current input of the meter. Edison (talk) 14:57, 9 July 2010 (UTC)
- No, man the zinc chloride bat are BRAND NEW, just tore the wrapper ! Jon Ascton (talk) 16:49, 8 July 2010 (UTC)
- Try some alkaline batteries again. If it works, there's your answer. If it doesn't, you've either got yourself a physical fault with the camera (see above with ohmmeter) or a software/hardware fault which would need to be inspected by the manufacturer. Presumably it's still under at least a years warranty? Regards, --—Cyclonenim | Chat 16:53, 8 July 2010 (UTC)
- Take an ohmmeter across the terminals in your camera. Is the resistance unusually low? 20.137.18.50 (talk) 16:38, 8 July 2010 (UTC)
- FWIW, I once bought a package of alkaline batteries and one of them was "dead" straight out of the package. -- Coneslayer (talk) 16:54, 8 July 2010 (UTC)
- Some zinc batteries will rapidly lose voltage under load - giving a false 'battery dead' reading. This happens usually when the zinc part of the battery is the case of the battery .. batteries using finely divided zinc with more surface area are less susceptable to this effect. This page [3] seems to say that zinc chloride cells also use the zinc can construction.
- Still a good idea to test the battery in something else (like a torch or motor).87.102.42.55 (talk) 17:03, 8 July 2010 (UTC)
- Check the instructions.. but I'm fairly sure that for digital cameras alkaline batteries (or better) are always recommended.87.102.42.55 (talk) 17:10, 8 July 2010 (UTC)
- Zinc chloride or Zinc carbon cells are usually prone leaking since the can disintegrates as the battery discharges. This page http://michaelbluejay.com/batteries/ concludes Absolute crap. Do not buy I've got to agree with that. - alkaline batteries are so cheap nowadays and according to the data on that site give more energy per currency unit.87.102.42.55 (talk) 17:16, 8 July 2010 (UTC)
- By the way if you can tell us what state you are in I'm sure someone will be able to give a good place to buy the most cost effective batteries.87.102.42.55 (talk) 17:22, 8 July 2010 (UTC)
- My digital camera will only work with alkaline batteries, and not with zinc chloride. I imagine the ZC ones do not provide enough power. 92.24.188.89 (talk) 18:32, 8 July 2010 (UTC)
- The battery voltage depends on the battery chemistry; alkalines have a slightly different voltage than NiMH rechargeables, and I suppose zinc chloride is slightly different as well. Also, digital cameras (unlike flashlights) draw a lot of current while taking photos and much less at other times, and different chemistries respond differently to that. All digital cameras that use AA batteries are designed to work with NiMH and alkaline, but I don't know about zinc. I'd advise buying some NiMH rechargeables, since they're the cheapest in terms of cost per photo, and also more convenient since they will give you far more photos per charge. -- BenRG (talk) 00:42, 9 July 2010 (UTC)


- Ok, Now we have brought home a pack of rechargeable Nippo batteries. I am pasting a picture Are these OK for use in my Nikon Coolpix L20 ?
- Note that they are 1.2 v not 1.5 v Jon Ascton (talk) 16:11, 10 July 2010 (UTC)
- Why did you buy them before finding out if they would work? Googling your camera shows it needs alkaline batteries, so rechargeable alkaline batteries will work but the battery life, especially after several recharges, will be worse than with standard alkaline AA batteries, but who cares, because you can recharge them. Regards, --—Cyclonenim | Chat 17:31, 10 July 2010 (UTC)
- Note alkaline ≠ AA. The camera needs AA batteries, but they don't have to be alkaline, and these aren't. The package says "Ni-Cd rechargeable". According to this spec page, the Coolpix L20 supports "Alkaline, NiMH, Oxyride or Lithium" batteries. NiCd isn't mentioned, but that might be only because it's obsolete (replaced by the superior NiMH). I don't necessarily trust these batteries because I haven't heard of the brand and I was under the impression that nobody made NiCd batteries any more. Also, there's wide variation in rechargeable battery capacity (quoted capacities range from 900 to 2700 mAh at least) and wide variation in charger speed (from <1 hour to many hours—the one you bought says 8 hours), so you should check those specs before you buy. -- BenRG (talk) 19:25, 10 July 2010 (UTC)
- Why did you buy them before finding out if they would work? Googling your camera shows it needs alkaline batteries, so rechargeable alkaline batteries will work but the battery life, especially after several recharges, will be worse than with standard alkaline AA batteries, but who cares, because you can recharge them. Regards, --—Cyclonenim | Chat 17:31, 10 July 2010 (UTC)
Burning polythene[edit]
What is the offensive and acrid gas resulting from the burning of polythene bags? Ethylene is just C2H4 after all. Androstachys (talk) 17:19, 8 July 2010 (UTC)
- Partially oxidised decomposition products ?? Are you sure it's polyethene - this might interest you http://www.boedeker.com/burntest.htm also see google books .. burn smells 87.102.42.55 (talk) 20:08, 8 July 2010 (UTC)
Polyethylene is a polymer....it's not the same as ethylene. Polyethylene has a lot of sigma bonds. Plus don't forget the presence of plasticisers and stabilisers. John Riemann Soong (talk) 21:58, 8 July 2010 (UTC)
- AFAIK the stench is due to pyrolysis and partial oxidation products: low-MW hydrocarbons, aldehydes, ketones, organic acids, that sort of stuff. (Pretty much the same stuff that makes diesel exhaust stink like a sonuvabitch.) FWiW 67.170.215.166 (talk) 03:01, 9 July 2010 (UTC)
- If you are getting an acrid gas perhaps you are burning polyvinyl chloride and making hydrogen chloride gas. Graeme Bartlett (talk) 11:10, 9 July 2010 (UTC)
Killer swan mom part 2[edit]

Ok, the cygnets hatched out June 12 or 13 and are already larger than the fattest mallard. Their parents are captive swans living in a half of a pond about the size of a soccer field (the other half houses another pair). There's also a pair of nesting common terns (the chicks will apparently fledge in a couple of days), and some poor mallards (of course these are wild, not captive) ... This female swan does not even look at the terns, but for some reason she kills the ducklings. They hide most of the day behind a rock ledge, but as soon as they venture in the open, the swan mom forgets her litter and charges at the ducklings. She reduced the older mallard litter to just one survivor, the other (younger) bunch still holds, but not for long. The swan daddy does not really care, he's obsessed with another male...
Question: do the females behave just as bad in the wild, or it's the price of captivity and tight space? East of Borschov 17:37, 8 July 2010 (UTC)
- The BBC's Springwatch programme this year featured a swan's brood being completely wiped out, mostly by such behaviour from other swans, so I would guess it's wild behaviour.--TammyMoet (talk) 20:11, 8 July 2010 (UTC)
- Swan attack (video) Cuddlyable3 (talk) 00:08, 10 July 2010 (UTC)
The effect of life on light shining through the atmosphere[edit]
Now that some crude images of extra-solar planets have been made, would the presence of life make any difference to the light that would pass through the atmosphere of a planet as it passes in front of its star? Could light reflected from the surface of the planet also be detected? 92.24.188.89 (talk) 18:47, 8 July 2010 (UTC)
- Yes, it is generally believed that living organisms will influence the atmosphere of a planet in ways that could be observed my studying the spectrum of light that has passed through the atmosphere (whether at the limb of the planet as it transits the star, or by reflection off the surface). Oxygen, for example, is not thought to persist well in planetary atmospheres, and must be replenished like it is by plants on earth. See, for example, Terrestrial Planet Finder: Detecting signs of life. -- Coneslayer (talk) 19:08, 8 July 2010 (UTC)
- Some astronomers are already trying to do that - so it's certainly not unreasonable. There have been several pronouncements about odd chemical imbalances in the atmospheres of Mars and some of the more interesting moons. For example, it was announced that the anomalous amounts of Methane in the atmosphere of Mars could only be explained by there being active microbial life there...until someone suggested that "serpentinization" could be responsible...and now we're not so sure again. The lesson to be learned here is that if we were to find a planet with a lot of oxygen (say), you can bet that "there must be life there!" will be the first conclusion - and then within a year or two of that, "well...we've thought of this other mechanism...so maybe not". What makes it difficult is that when you don't know the composition of the rocks - or almost anything else about the planet other than it's mass and orbital parameters - it's very tough to come up with a definitive answer. SteveBaker (talk) 21:40, 8 July 2010 (UTC)
I wonder how you would seperate the 'signal' of the light shining through the atmosphere of an extra-solar planet from the overwhelming 'noise' of the starlight? 92.24.181.157 (talk) 10:55, 9 July 2010 (UTC)
- Conceptually it's not that hard... obtain "baseline" spectra of the star when the planet is not transiting, and then take spectra during the transit and look for "new" absorption features (in excess of the overall decrease of light from the planet's opacity) corresponding to gasses of interest in the atmosphere. In practice, though, it's a challenging measurement, because the absorption features will be quite weak. You have to be very careful about understanding your instrumentation, along with any temporal variations in the equipment, Earth's atmosphere (if using a terrestrial telescope), or the star's light. We are getting there, though. See HD 189733 b; footnote 18 has an arXiv link where you can get a PDF of a paper with Spitzer Space Telescope spectra that are said to show water and methane in the planet's atmosphere. This is a Jupiter-sized planet, though... a small terrestrial planet will be considerably harder. (But I'm 33 years old, and when I was an undergraduate, the discovery of extrasolar planets at all was the Big New Thing. We keep moving forward, and we'll get there.) -- Coneslayer (talk) 17:39, 9 July 2010 (UTC)
- It is possible to detect the spectra of certain greenhouse gases in the atmosphere of a planet, for example methane which could be produced by life. See Extrasolar planet#Temperature and composition. ~AH1(TCU) 14:55, 10 July 2010 (UTC)
mythbusters nitro ram recoil.[edit]
In a mythbusters episode I saw they used a nitro ram to power a fist that knocked a dummy around, but the pipe that held the fist was just on a stand. So in theory someone could hold the pipe with the fist in and fire it off. So where does the equal and opposite force go that projects the first forward so fast. Does it work something like a recoiless rocket launcher, is there an apposing force? or is the recoil just applied elsewhere?
Thanks guys —Preceding unsigned comment added by 86.129.209.180 (talk) 20:43, 8 July 2010 (UTC)
- There is always an opposing force. I'm not familiar offhand with the specific episode, but from your description it sounds like the device is powered by pressurized gas. In that case, the gas can't escape out the fist end of the device, instead escaping out the back -- that is, a rocket. The one force is on the (closed) fist end (propelling the device, per Newton's second law), the other on the (open) exhaust end. Whether the system is recoilless is incidental to the way the forces conceptually work; either way it's a rocket-type system. — Lomn 21:01, 8 July 2010 (UTC)
- The stand was probably just strong enough to withstand the recoil, so it is the Earth that actually recoils. A person can also throw a punch without falling over. --Tango (talk) 21:04, 8 July 2010 (UTC)
acoustic levitation and spiderman[edit]

If objects can be levitated by soundwaves, then is it at least in theory possible to make an acoustic pulse weapon that can knock people down or objects, kind of like how shocker does it in spiderman. Or is there some kind of acoustic cut off point, or limit it can reach in terms of impulse .
thanks and sorry the question is kind of stupid. I was just wondering is all. —Preceding unsigned comment added by 86.129.209.180 (talk) 21:32, 8 July 2010 (UTC)
- I think the frog you're thinking of (yes, I know you're thinking about a frog) is the one pictured, which is being subjected to magnetic levitation. Comet Tuttle (talk) 21:41, 8 July 2010 (UTC)
No talking about acoustic levitation.
Thanks —Preceding unsigned comment added by 86.129.209.180 (talk) 21:50, 8 July 2010 (UTC)
- Can objects be levitated by ordinary soundwaves? It seems unlikely. Sound consists of alternating bands of high and low pressure - the air itself doesn't move bodily outwards from the source - it merely moves back and forth with the motion propagating outwards by no part of the air moving by very much without moving back again. If an object were to get a little 'push' as the sound wave compresses air up next to it - then a half-cycle later, the low pressure part of the wave would suck it right back again...so at best, you could only make your target vibrate (which might be enough to 'kill' it - but not enough to move it physically).
- The "acoustic levitation" trick relies on non-linearities in these properties of the air at extremely high pressures - I think it's more likely that you'd vibrate them to death before you knocked them over. Our article on acoustic levitation says that there is a practical limit of a kilogram or so that can be levitated in this way - but that's not enough to knock over a person.
- There are toys (called things like Air bazooka) which send a single large pulse of compression wave outwards...and giant versions of those like this are able to knocks over stuff like empty soda cans - but that's not like a continuous sound wave, material actually travels along the path of the compression - you can see this happen when people use such machines to shoot smoke rings. It's generally a bad idea to try to learn physics from spiderman comics! SteveBaker (talk) 21:56, 8 July 2010 (UTC)
- I've heard there's a variant of the Brown note that makes you lose your balance and actually exists. I might be wrong about that second part. 67.172.112.226 (talk) 22:06, 8 July 2010 (UTC)
Of course it's possible to make an acoustic pulse that will knock people down: just set off a suitably sized explosion. If you want to do it with a non-explosive device, that'd be harder. --Anonymous, 16:30 UTC, July 9, 2010.
- No - an explosion isn't like sound. It's large amounts of gaseous products from the rapid combustion of the explosive - that bodily moves outwards. Sound is a back-and-forth motion - which is why this is a problematic suggestion. At any given point along the path of a sound wave, the pressure alternates higher and lower than the 'ambient' air pressure. Which exerts alternating outwards and inwards forces on whatever it impacts. With an explosion, a few kilogrammes of gas (which is a LOT) move outwards - bodily in an effort to equalize the pressures everywhere. The pressure at any given point goes up as the shock wave goes by - and then gradually falls back to ambient. It never gets below the ambient air pressure so it never 'sucks' the object it hits back towards the source...but that's precisely what DOES happen with sound - and ordinarily the outward 'push' is immediately and perfectly counteracted by the inward 'suck'. Explosions only 'push'. The physical motion of that gas at great speed is what imparts the net force onto the object - and the force is ONLY away from the source. The only way for 'sound' to do this is for some very extreme thing to happen where the sound is so spectacularly loud sound that the air pressure drops to zero in the low pressure parts (and can therefore drop no further) - or the high pressure parts hit some non-linear effect within the gas (like it liquifies or something). That produces asymmetry between high and low parts of the sound wave which really can produce a net outward force. But that's crazy loud sound! SteveBaker (talk) 03:44, 10 July 2010 (UTC)
- Good point. However, you're talking about the blast and I was thinking of the shock wave, which is felt much farther away. I'm not sure to what extent, if any, it involves back-and-forth motions like ordinary sound, but I think the fact that it propagates through the air as a wave qualifies it as sound even if it does move supersonically. --Anon, 05:13, July 10, 2010.
Forest terminology — non-coniferous?[edit]
Can't for the life of me remember if there is a term to describe forests consisting only of leafy trees, as opposed to those containing pines, furs, and so on. Is there such a term that I can use in contrast to coniferous? Spent the last hour or so reading about trees to no avail. BigNate37(T) 22:07, 8 July 2010 (UTC)
- Deciduous or hardwood. Not that all deciduous trees are hardwood, and not all hardwood is hard (eg balsa) CS Miller (talk) 22:12, 8 July 2010 (UTC)
- And not all hardwoods are deciduous. Googlemeister (talk) 13:25, 9 July 2010 (UTC)
- Broad-leaved might be a better bet. Alansplodge (talk) 23:18, 9 July 2010 (UTC)
- And not all hardwoods are deciduous. Googlemeister (talk) 13:25, 9 July 2010 (UTC)
dehydrated yogurt[edit]
will dehydrated yogurt spoil quickly if left sealed in a package on the shelf and not refrigerated? —Preceding unsigned comment added by 71.137.244.115 (talk) 22:20, 8 July 2010 (UTC)
- Please clarify what you mean by "dehydrated yoghurt". That could mean half a dozen things, and I don't feel like trying to give all the possible answers (even if I could!). Looie496 (talk) 22:50, 8 July 2010 (UTC)
- Dehydrated yoghurt is probably similar to dehydrated milk that keeps indefinitely in powdered form without refrigeration, and can be carried as part of a trail snack. Cuddlyable3 (talk) 10:24, 9 July 2010 (UTC)
Evolution and entropy[edit]
Why isn't the accumulation of beneficial mutations a violation of entropy? Is it because we assume that accumulations of neutral and disadvantageous mutations also occurs, but that the former is silent and the latter causes those involved to perish, leaving only the beneficial mutation accumulators to seem as though they are violating this concept? DRosenbach (Talk | Contribs) 23:56, 8 July 2010 (UTC)
- I don't know whether they help, but we have articles on entropy and life and negentropy. ---Sluzzelin talk 00:04, 9 July 2010 (UTC)
- (edit conflict - the article negentropy is what I'm describing here roughly) I'm not really answering the evolution/dna part of your question - but stating that (all) living creatures are machines that 'violate entropy' that is they internally decrease or maintain their entropy whilst converting other material (eg food) to products that have much higher entropy.
- In the same way a machine that sorts M&Ms or Smarties into different colours, then destroys all colours except blue doesn't violate entropy (even though the entropy of the sweets has decreased by increased ordering) - since the machine requires a source of energy to operate (that energy being converted ultimately to heat - and thus to increased entropy).87.102.42.55 (talk) 00:08, 9 July 2010 (UTC)
- (after some really weird Wiki bugs) Ultimately the life on Earth is driven by the energy we get from the Sun, so the global entropy of the Solar System is probably not decreasing :) . That being said, a relation between thermodynamic entropy and life has been researched on and off for about 100 years now. We have some articles on Entropy and life, but they are little more than stubs. I haven't found a good book on the subject yet, either. --Dr Dima (talk) 00:13, 9 July 2010 (UTC)
- The second law of thermodynamics only applies to closed systems. Earth's biosphere is not a closed system, because of the influx of energy from the sun. Even if it were, the accumulation of mutations wouldn't automatically violate the second law, because it only requires that the total entropy of a system increase, not that the entropy of every part individually increase. Looie496 (talk) 00:51, 9 July 2010 (UTC)
- (ec) You have a mistaken understanding of the Second law of thermodynamics. It says merely that entropy increases over time in closed systems. "Closed systems" is important here—it means that additional energy is not entering into the system. You can lower the amount of entropy in an open system. I do it all the time when I clean my office, moving things from chaos towards order. It just requires energy. In the case of my room, I bring the energy in through a chain that ultimately traces itself back to the Sun. Life is itself a giant lowering of entropy—it is organization, self-organization. It requires huge amounts of energy to do this. It doesn't violate the second law of thermodynamics, though, because the system is not closed. In the case of genetics, the entropy is kept at a reasonable level because of natural selection itself—it weeds out the total chaos, the total duds, and channels that "energy" (which is now getting extremely metaphorical) towards the beneficial genes. --Mr.98 (talk) 00:53, 9 July 2010 (UTC)
- Victor Stenger's book, God the Failed Hypothesis has a good treatment of this subject as it applies to life and the whole universe.. Since after you get past "life", why should there be planets and suns and galaxies at all if everything started from nothing. I'm not starting the argument, i'm just saying his book explains it with science and all quite well. Vespine (talk) 01:35, 9 July 2010 (UTC)
- This and many other fallacious arguments espoused by proponents of creationism (and its dressed-up cousins) are touched on in our article on intelligent design. This page provides a thorough explanation of the flaws in the 'evolution violates the laws of thermodynamics!' argument of intelligent design proponents. TenOfAllTrades(talk) 01:53, 9 July 2010 (UTC)
- Also, I think it's debatable whether or not life, or the evolution thereof, would necessarily increase entropy at all. I think increased amounts of life certainly will, and more complex machines will, but how is a human more complex of a machine than a cow? Surely the cow has more moving parts - look at all that chewin' and digestin'! SamuelRiv (talk) 09:46, 9 July 2010 (UTC)
- I would suggest that evolutionary radiation - the diversification of a small number of species into many - represents an example of an increase in entropy consistent (more precisely, inherent to) evolution. Looked at this way, evolution is perfectly Second Law compliant! – ClockworkSoul 22:49, 9 July 2010 (UTC)
Entropy applies to 'closed systems' - the earth isn't a closed system because we get sunlight coming in - which is nice low-entropy stuff - and it winds up as low grade infra-red radiation - which is high-entropy stuff. Think of it like this - you have a room with a robot and a whole bunch of children's building blocks scattered all over everywhere. If you supply power to the robot, it can pick up the blocks and stack them neatly - which reduces their entropy by removing chaos and adding 'order'. But wait! That can only happen if you supply external power to the 'system' by plugging in the robot - it's not a 'closed system'. If you don't plug in the robot, nothing happens and entropy doesn't decrease. However, when the robot is plugged in, somewhere a power station is converting organized, low entropy coal into chaotic high entropy CO2 in order to make the electricity to run the robot. The amount of entropy created by the power station is much more than the entropy removed by the robot. In a 'closed system' comprising (1) a pile of coal, (2) a power station, (3) a robot and (4) the building blocks, the coal gains entropy at a higher rate than the building blocks lose it - so the TOTAL entropy of the closed system increases over time - just as the laws of thermodynamics tell us they must. With animals (rabbits maybe), the local lowering of entropy in their bodies is more than compensated for by the way they consume low entropy foods like carrots maybe and produce high entropy heat and poop. The carrots, in turn, took high entropy CO2, water and dirt and 'organized it' into low-entropy carrot - but only at the cost of turning low entropy sunlight into high entropy infrared. Just as with the robot and the power station, we have low entropy sunlight turned into high entropy IR faster than the carrots and the rabbits can keep their low entropy existence intact. So it's safe to say that the small amount of local lowering of entropy due to evolution is dramatically outweighed by the additional entropy that process created. SteveBaker (talk) 03:36, 10 July 2010 (UTC)

