Wikipedia:Reference desk/Archives/Science/2009 January 28
| Science desk | ||
|---|---|---|
| < January 27 | << Dec | January | Feb >> | January 29 > |
| Welcome to the Wikipedia Science Reference Desk Archives |
|---|
| The page you are currently viewing is an archive page. While you can leave answers for any questions shown below, please ask new questions on one of the current reference desk pages. |
January 28[edit]
Can one guess the mass of a dice?[edit]
Suppose we have a cube with labeled faces (e.g., one of those objects called dice), with (possibly) inhomogeneous mass. Given the probabilities of the outcomes 1,..,6, (say that we have thrown it so many times that we agree on them), can we at least locate the baricenter? Or conversely: besides the symmetric case, is there a distribution of mass in a cube for which we can guess the frequencies of the outcomes? (Disclaimer: I am not planning to cheat people) --pma (talk) 00:43, 28 January 2009 (UTC) hopefully fixed
- There is no such thing as "a dice". --Trovatore (talk) 00:58, 28 January 2009 (UTC)
- You can make a guess, but only within certain statistical parameters. Note that casino dice have the pips filled so as to maintain symmetry of mass. — Lomn 01:02, 28 January 2009 (UTC)
He never did say "a dice", he quite correctly and grammatically said "one of those objects called dice" (objects dice plural). Don't be pedantic and wrong. Rotational (talk) 13:40, 29 January 2009 (UTC)
- He did say (correctly) "a dice", but has since edited the original question to avoid confusing Americans. DuncanHill (talk) 14:57, 29 January 2009 (UTC)
My mistake......sorry Rotational (talk) 17:19, 29 January 2009 (UTC)
- What Trovatore is saying is that "dice" is the plural form of "die". Saying "a dice" is like saying "a cars" or "a books", it doesn't really make sense. -- Mad031683 (talk) 01:21, 28 January 2009 (UTC)
- Surely the OP means a die. Surprised no one linked this--GreenSpigot (talk) 01:51, 28 January 2009 (UTC)
- OK die redirects to dice. But die is the singular.--GreenSpigot (talk) 01:57, 28 January 2009 (UTC)
- We're not really supposed to correct the OP's grammar/vocabulary. If you can't answer the question...don't. SteveBaker (talk) 02:19, 28 January 2009 (UTC)
- Correction in this case gives the OP more of a chance of finding what (s)he was looking for: therefore it is legitimate.--GreenSpigot (talk) 02:27, 28 January 2009 (UTC)
- Dice is perfectly acceptable as a singular. DuncanHill (talk) 02:32, 28 January 2009 (UTC)
- No, it isn't. It sounds seriously uneducated. --Trovatore (talk) 02:34, 28 January 2009 (UTC)
- Dice is the normal singular, and the old singular die is confined to a few fixed phrases and certain mathematical contexts. DuncanHill (talk) 02:43, 28 January 2009 (UTC)
- No, it isn't. A dice is substandard English, like a bacteria or a criteria. Absolutely unacceptable in formal writing. --Trovatore (talk) 02:45, 28 January 2009 (UTC)
- Will you write and tell the OUP that or shall I? DuncanHill (talk) 02:47, 28 January 2009 (UTC)
- The Brits do seem to see these things differently. They're wrong, of course. But I'd be surprised about Oxford; I'd need to see evidence there. --Trovatore (talk) 02:51, 28 January 2009 (UTC)
- Edmund Weiner, Andrew Delahunty, Oxford Guide to English Usage, 2nd edition 1993, page 130. That do you? DuncanHill (talk) 02:55, 28 January 2009 (UTC)
- What does it say, exactly? --Trovatore (talk) 02:56, 28 January 2009 (UTC)
- "dice is the normal singular as well as the plural (one dice, two dice); the old singular, die, is found only in the die is cast, straight (or true) as a die, and in mathematical discussions, e.g. Rolling a die will generate a string of random numbers." Further down the same page it has "die (noun); see dice." DuncanHill (talk) 03:00, 28 January 2009 (UTC)
- Boy, good thing we Americans are around to save the language. Y'all aren't taking care of it at all.
- Anyway, you should be aware that a dice is seriously grating on American ears. In shared situations, if you can't bring yourself to say a die, it would be better to rephrase. --Trovatore (talk) 03:04, 28 January 2009 (UTC)
- (ec)God forbid Americans should have to put up with English as She is Spoke in England :) David Crystal in The Cambridge Encyclopedia of the English Language, CUP, 1995, makes a similar point to the Oxford, at page 201. DuncanHill (talk) 03:08, 28 January 2009 (UTC)
- Both 'die' and 'dice' are unacceptably vague here, and 'cubic die' sounds unnatural to my ears. The object in question is a d6. Algebraist 03:07, 28 January 2009 (UTC)
- I second d6. All Dungeons and Dragons players know that the only acceptable way to reference dice is to day 'd' followed by the number of sides on the dice (die) in question, most commonly d20. -04:00, 28 January 2009 (UTC) —Preceding unsigned comment added by Pete5x5 (talk • contribs)
- "dice is the normal singular as well as the plural (one dice, two dice); the old singular, die, is found only in the die is cast, straight (or true) as a die, and in mathematical discussions, e.g. Rolling a die will generate a string of random numbers." Further down the same page it has "die (noun); see dice." DuncanHill (talk) 03:00, 28 January 2009 (UTC)
- What does it say, exactly? --Trovatore (talk) 02:56, 28 January 2009 (UTC)
- Edmund Weiner, Andrew Delahunty, Oxford Guide to English Usage, 2nd edition 1993, page 130. That do you? DuncanHill (talk) 02:55, 28 January 2009 (UTC)
- The Brits do seem to see these things differently. They're wrong, of course. But I'd be surprised about Oxford; I'd need to see evidence there. --Trovatore (talk) 02:51, 28 January 2009 (UTC)
- Will you write and tell the OUP that or shall I? DuncanHill (talk) 02:47, 28 January 2009 (UTC)
- No, it isn't. A dice is substandard English, like a bacteria or a criteria. Absolutely unacceptable in formal writing. --Trovatore (talk) 02:45, 28 January 2009 (UTC)
- Dice is the normal singular, and the old singular die is confined to a few fixed phrases and certain mathematical contexts. DuncanHill (talk) 02:43, 28 January 2009 (UTC)
- No, it isn't. It sounds seriously uneducated. --Trovatore (talk) 02:34, 28 January 2009 (UTC)
- English is defined solely by how it is used (we don't have an equivalent of the Academie Francaise, for example), dictionaries just report what is used. If enough people make the same mistake, as in this case (or the case of "begging the question", for example), it becomes correct. --Tango (talk) 15:10, 28 January 2009 (UTC)
- Dice is perfectly acceptable as a singular. DuncanHill (talk) 02:32, 28 January 2009 (UTC)
- Correction in this case gives the OP more of a chance of finding what (s)he was looking for: therefore it is legitimate.--GreenSpigot (talk) 02:27, 28 January 2009 (UTC)
- An easier way than relying on random movements and outcome while rolling the die/dice would be to build yourself a contraption where you could stand/clamp the thingy in question in by 2 diagonally opposed corners [1] and give it a controlled push with a known and repeatable force and speed. Dice with centered mass should spin at the same rate and slow down to a stop within the same time no matter which corners you used. Dice instead of die is common because most of us encounter them before we get to school. (Even in the US.) I learned about a die for casting before I found out it was also (officially) the singular for dice.76.97.245.5 (talk) 03:54, 28 January 2009 (UTC)
Thank you everybody! So, I understand that the singular of dice is not so used, like in latin. Maybe I tried the Language desk for the mechanical side of the question...--pma (talk) 09:36, 28 January 2009 (UTC)
- Some interesting discussions on two subjects!! The grammar bits were especially entertaining, but neither the title nor the question referred to "a dice". Bazza (talk) 13:41, 28 January 2009 (UTC)
- Yes it did. It's been changed. Algebraist 13:44, 28 January 2009 (UTC)
- And now I'have re-corrected at least the title so as to keep memory of the lucky occasion that gave origin to this debate. Note that, as a non-native english speaker, I am really glad to be corrected. In fact, I started writing here also to improve my english. And, of course, because I believe in sharing the knowledge with people from different countries. pma (talk) 14:39, 28 January 2009 (UTC)
- Yes it did. It's been changed. Algebraist 13:44, 28 January 2009 (UTC)
- Determining the centre of mass for the die by how it rolls is probably impossible in practice. The actual way to do it is to attach a string to the die to a bunch of points, one point at a time. The centre of mass will lie along the line that extends downward from the string (to maintain no torques on the die). Do this twice and the intersection of the two lines reveals the location of the centre of mass, but in practice, you're best to do several measurements to beat down the experimental error. WilyD 14:45, 28 January 2009 (UTC)
If we could all just use impeccable grammar, it would be a pair o' dice. Edison (talk) 16:20, 28 January 2009 (UTC)
- Thanks Wily. Yes, we can determine geometrically the baricenter, but what I had in mind is another issue: is there a relation between the frequencies and the position of the baricenter? Roughly, the dice will stop more often with their baricenters in the lower position; I just wonder how this can be made quantitative. I had in mind a kind of approach via statistical mechanics and... no matter. I found interesting the linguistic aspect, and even more the anthropological one ;) --pma (talk) 17:42, 28 January 2009 (UTC)
- Err, realistically, you'd never work out the relationship theoretically. Depends on the details on the friction in the die roll, the non-cubicity of the die, many unpleasant bits. You could make a simplified model, but the devilish details would cause me, a theorectical physcists to say "This problem is impossibe" - the easiest way to do it would be a bunch of dies with known baricentres, an undergraduate and some money from NSERC (or the NSF or whatnot). WilyD 18:13, 28 January 2009 (UTC)
- Ok, that's what I also suspect; too many details are to be fixed. Now, trying to answer by myself, a simple model is: an cubic die with sharp edges, rolling under the effect of impulses randomly distributed. The six positions are local minima for the energy, and a 90 degrees rotation from one face (i) to another (j) needs a small amount of energy, δij -it's how much the baricenter has to be raised. This should give a transition probability pij for any pairs of adjacent faces; pij is related to δij according to the distribution of the random impulses. Now we have a Markov chain and I may think that the corresponding stationary distribution is the probability distribution for the stopping positions. The idea is that at a certain moment the die just decides to stop for reason of his own (otherwise, in alternative one may think of a slowly decaying energy, acting till the die can't move any longer). Anyway, my interest about it is just linked to dinner conversations; I'm not a physicist nor a probabilist and can't say how fool is this picture. pma (talk) 19:59, 28 January 2009 (UTC)
- (Thanks for hiding the off topic!) The problem is there are even more details than just the dice itself. Even consider the initial position as random, the initial hight and spin have an impact. If it drops from a large hight with little spin, it is much more likely to make it into some equilibrium position before hitting the ground. It also depends on how much the die bounces on the surface it is being dropped. A lot of bouncing would again lead to more randomness and less predictability which would mean you'd have to use a die with a weight even more from the center. If you wanted to make a situation where the dice would land more predictably I may suggest dropping into a large tank of undisturbed water with sand at the bottom. The large decent would give it enough time to reach its equilibrium state and its landing would be slow because of the water so it'd probably just stick into the sand the same direction that it was falling. Even for a dice that was just off a little, you may find that you get the same number almost every time in this situation. Anythingapplied (talk) 21:40, 28 January 2009 (UTC)
- Yes, the way it's thrown is clearly relevant. For the slow die in water that you are describing, the probability to land on a face should be proportional to the solid angle subtended by that face at the baricenter, I think. Anyway, I would expect just a rough correspondence between a true die and a mathematical model. Thanks again pma (talk) 22:10, 28 January 2009 (UTC)
- I think you're model is solid. If you use some sort of machine to throw dice consistantly and fit variables to the model, I think it'd work quite well for a controlled situation. If you could use a high speed camera to record the initial side that hit the ground, the number of bounces, and all the intermediary sides to be face up when making contact with the ground you'd be able to fit your variables with much higher relaibilty and far fewer experimental throws. Determining what probability distrabution to use for the amount of initial energy would be the most difficult part in my opinion. Anythingapplied (talk) 22:23, 28 January 2009 (UTC)
- Yes, the way it's thrown is clearly relevant. For the slow die in water that you are describing, the probability to land on a face should be proportional to the solid angle subtended by that face at the baricenter, I think. Anyway, I would expect just a rough correspondence between a true die and a mathematical model. Thanks again pma (talk) 22:10, 28 January 2009 (UTC)
- (Thanks for hiding the off topic!) The problem is there are even more details than just the dice itself. Even consider the initial position as random, the initial hight and spin have an impact. If it drops from a large hight with little spin, it is much more likely to make it into some equilibrium position before hitting the ground. It also depends on how much the die bounces on the surface it is being dropped. A lot of bouncing would again lead to more randomness and less predictability which would mean you'd have to use a die with a weight even more from the center. If you wanted to make a situation where the dice would land more predictably I may suggest dropping into a large tank of undisturbed water with sand at the bottom. The large decent would give it enough time to reach its equilibrium state and its landing would be slow because of the water so it'd probably just stick into the sand the same direction that it was falling. Even for a dice that was just off a little, you may find that you get the same number almost every time in this situation. Anythingapplied (talk) 21:40, 28 January 2009 (UTC)
- Ok, that's what I also suspect; too many details are to be fixed. Now, trying to answer by myself, a simple model is: an cubic die with sharp edges, rolling under the effect of impulses randomly distributed. The six positions are local minima for the energy, and a 90 degrees rotation from one face (i) to another (j) needs a small amount of energy, δij -it's how much the baricenter has to be raised. This should give a transition probability pij for any pairs of adjacent faces; pij is related to δij according to the distribution of the random impulses. Now we have a Markov chain and I may think that the corresponding stationary distribution is the probability distribution for the stopping positions. The idea is that at a certain moment the die just decides to stop for reason of his own (otherwise, in alternative one may think of a slowly decaying energy, acting till the die can't move any longer). Anyway, my interest about it is just linked to dinner conversations; I'm not a physicist nor a probabilist and can't say how fool is this picture. pma (talk) 19:59, 28 January 2009 (UTC)
- Actually, I think one could get an approximate answer that while far from complete might none the less be interesting. If you watch a die, then at any given instant there is nearly always one side that is more approximately "up" than the others, and the die will either settle on that side or rotate to another. The question of whether it rotates to another side is dependent on whether it has enough angular momentum to carry its center of mass against gravity across a pivot point. A higher center of mass is easier to flip than a lower one. If one chooses to consider only the "last flip", when the angular momentum of the die is sufficient to rotate over once more but no further, one should be able to state roughly how much easier it is to flip the die in one configuration versus another based on the position of that center of mass. If one assumes that the last part of the roll, when the die is about to settle, is the most important, then this would give a rough way of estimating. Obviously there are a lot of other details that could modify that conclusion, but I think there is nonetheless a tractable (though still complicated) starting point for making such estimates. Dragons flight (talk) 22:35, 28 January 2009 (UTC)
Hey! Someone deleted my answer!! (restoring it) I've been thinking about this for the past day or so while all of the babble about die/dice was going on. I think we don't have enough information. Think about the mechanisms by which a 'weighted' dice ends up being 'unfair':
- If it were being tossed in a vacuum, then while it was in the air, it would rotate about it's center of gravity (which would be off-center) but that would only affect it's rate of rotation because of the changed moment of inertia of an off-center center of gravity compared to a dice with the CofG in the middle. So the initial force imparted to roll the dice would have a different resulting spin rate - but once launched, it would still land (essentially) randomly depending on the precise speed and height it was thrown from...and (of course) what orientation it was in at the start of the toss. But a dice with a heavy weight just under the '1' face wouldn't preferentially come up '6' for this reason.
- In the presence of air resistance, there would be a tendency for it to slowly stop rotating and to fall 'heavy-side-down'. But the degree to which that happens would have to depend on the lightness of the body of the dice. Imagine something like a shuttle-cock - those lightweight feathers result in it's flight rapidly stabilising to a 'heavy-end-first' approach. But if you imagine a 4 ton solid steel dice with a similarly off-center weight, you'd imagine that air resistance would be negligable. So this tells us that the weight, size, aerodynamics of the dice will affect the degree to which the thing will or will not tend to stabilise.
- Then, when the dice hits the ground and starts rolling there will be the question of whether the corner of the dice has enough friction to allow it to flip over onto the next face instead of merely sliding along the table without rolling. Offsetting the weight further from the point where the friction is applied gives it a greater turning moment when the weight is high up - and reduces the turning moment when the weight is at the bottom - so that's going to increase the probability of it ending up heavy-side down rather than managing to rotate another 90 degrees. The degree to which this effect works depends on how steeply the dice is dropped (if it's vertical - then this has little effect - if it's rolled horizontally - such as as a casino 'craps' table - then it's very significant).
So here's the problem. The degree to which weighting the dice affects the outcome depends on (at least) the air resistance, the moment of inertia of the unweighted version of the dice, the frictional forces between dice and table and the angle from which the dice is thrown. All of those things will alter the probability of the dice to be 'unfair'. If the degree of unfairness (statistical irregularity) depends on something OTHER than the placement of the weight in the volume of the dice - then you can't use statistical methods to determine how far the weight is from the center. You almost certainly can determine the direction in which it's displaced because that's just the degree of asymmetry in the statistics. But the AMOUNT of displacement would require knowing an awful lot about the detailed physics.
SteveBaker (talk) 00:38, 29 January 2009 (UTC)
Very clear. By the way, now I understand why casino dice have sharp edges: one good reason is that rounded corners would make it easier to stop on the lower position of the baricenter, if it's not perfectly centered - as extreme case, a ball would do it certainly. --pma (talk) 07:36, 29 January 2009 (UTC)
- Yes - I wondered the same thing. It does make sense that square-cornered dice would be somewhat less prone to weighting - but on the other hand, they might be more prone to not being so random by virtue of not rolling so well. If you imagine some kind of dice that would somehow not roll at all (maybe it has spikes on the corner that dig into the table!) - it would be pretty easy to cheat by tipping it out of your hand - or perhaps the dice cup - in some kind of carefully controlled manner. An almost spherical dice seems less problematic in this regard. Going back to the 'dungeons & dragons' dice - a 20 sided icosahedral dice rolls forever - but a d4 (which is a tetrahedron) really doesn't roll at all. I have a set of d4 dice that are cylindrical 8-sided things with each number printed on them twice. They are much nicer to play with. I often suspect people of kinda gently tipping the tetrahedral ones onto the table to get the number they want. However, that may not be an issue for casino's where they make you roll the dice the entire length of that L-O-N-G table. SteveBaker (talk) 19:12, 29 January 2009 (UTC)
Backwards FET operation[edit]
Can I use a FET backwards to generate the square root of the drain current by looking at the Vgs?--GreenSpigot (talk) 02:05, 28 January 2009 (UTC)
- It won't work if you just use the FET on its own, which is what I think you mean. The FET won't 'know' that you want the Vgs to vary with the drain current. You can, however, put the FET in the feedback loop of an amplifier and reverse its operation that way. There's an example here. --Heron (talk) 09:55, 28 January 2009 (UTC)
- Yes but the opamp in that cct is only used to generate a drain (actually source current in that config) current proportional to the signal voltage. Id rather not use an op amp as Im considering very high frequencies. So if I was to force a drain current and use the developed Vds to act on the gate as feedback, do you think it may work?--GreenSpigot (talk) 13:33, 28 January 2009 (UTC)
- In essence, you can't "reverse" the FET but you can ask the question "What voltage, when squared, would equal this voltage?" - and that is the answer you want. Hence User:Heron's idea of using a feedback approach. So (effectively) you compare the output of the FET to the voltage you are trying to take the square root of - and adjust the input of the FET up or down until they match. (Or if you don't need to do it too quickly you could use a $5 microprocessor that has A/D and D/A and do it in software!) SteveBaker (talk) 14:01, 28 January 2009 (UTC)
- Its ok I modelled it on spice and it works.--79.75.56.52 (talk) 17:14, 28 January 2009 (UTC)
- BTW thats SPICE--GreenSpigot (talk) 01:13, 29 January 2009 (UTC)
intensity light[edit]
SteveBaker, You said if I'm on Mars I won't notice the vermilion color becasue my eyes is in vermilion color, and the color I'll see is just tranparent light. Then why on Earth, on foggy day the I still notcie white, I see sky everyday, they always look azure (light blue). The yellow (signal light), I think way inside, humans can notice the yellow colour. But on Saturn/Titan, the problem is the light we get is 1/100 that of Earth, that is only light of a thunder hit our house at night. If I orbit around looking down, would Saturn look almost black? When I descend in Titan's atmosphere, you said I won't see the orange color because orange colour is in my eyes. I thought the color won't burn my eyes blind, just turn my color vision off? is strong color not rich just burn off vision?--69.226.46.118 (talk) 02:00, 28 January 2009 (UTC)
- You seem to be referring to this thread. Be aware that if you show up here continuing a discussion from a week ago, and speaking directly to an unspecified person (who are you talking to, anyway?), most people here will wonder what on earth you're talking about. Algebraist 03:12, 28 January 2009 (UTC)
- I think it's a steganographic message of some sort. Taking 1=color and 0=colour, it comes out 111010111. Not very good steganography — low information density; easily broken. Reminds me of this. --Trovatore (talk) 03:21, 28 January 2009 (UTC)
- To avoid confusion the OP modified the post, original was here [2]. To the OP, it is usually considered poor form to modify your post in such a way that you make proceeding discussion meaningless. You should at least mention it was modified or better still, if modifying your post will confuse the situation, just reply to your post with the additional information Nil Einne (talk) 11:16, 28 January 2009 (UTC)
- Trova, What??--69.226.46.118 (talk) 04:26, 28 January 2009 (UTC)
- He's referring to the fact your original message was fairly cryptic. Nil Einne (talk) 11:16, 28 January 2009 (UTC)
- Unfortunately, he feels very bad when he finds mis-spells and typos, and goes crazy. Usually these are results of a severe education... --pma (talk) 11:55, 28 January 2009 (UTC)
- (Background: The OP had been asking a lot of questions - with a lot of follow-ups about the general topic of the color that planets would seem to be if you were really there. (S)he started asking more of these questions over on my UserTalk: page - and I requested that they not be posted there - but here, where they belong. IMHO, between several L-O-N-G answers here - and more on my UserTalk: the question has already been fully answered and there is little (if anything) more to be said on the subject. IMHO, the OP should carefully read the previous replies and desist from further posting on the subject until all of that material has been properly absorbed. At any rate - I've had enough.) SteveBaker (talk) 13:54, 28 January 2009 (UTC)
- What matters isn't what colour the things you're seeing are, but rather what colour the ambient light is. On Earth, the ambient light is almost always white (that's why our eyes see the frequencies they do) - while the sky is blue, the sun is yellow, and they add together to make white (in fact, the light from the sun started out as white and was split into blue and yellow by the atmosphere). On Mars, the ambient light is going to be more red because the dust in the atmosphere absorbs other colours (this is different from the scattering that makes our sky blue - that just makes different colours seem to come from different places, it doesn't absorb anything so the result is still white), and your eyes would very quickly get used to that and you wouldn't notice (if it was too red you would notice because certain colours (eg. blue) would appear extremely dark, even black, but on Mars it wouldn't be that severe). --Tango (talk) 15:05, 28 January 2009 (UTC)
- This reminds me of the Goethe vs. Newton dispute on the theory of colours... even Newton had enough of it, at a certain point ;) pma (talk) 18:18, 28 January 2009 (UTC)
- Newton avoided the worst of that dispute by dying two decades before Goethe was born. Algebraist 15:13, 29 January 2009 (UTC)
- This reminds me of the Goethe vs. Newton dispute on the theory of colours... even Newton had enough of it, at a certain point ;) pma (talk) 18:18, 28 January 2009 (UTC)
Feed the sailors with carrots so they'll have better eyesight.[edit]
The Japanese battleship Yamato was the last serious and also the largest navy battleship ever built. It was sunken by U.S. navy pilots in 1945. In 1941, it had a very traditional battleship vs. battleship configuration. In 1944, they removed two 155 mm turrets and installed 138 25 mm AA guns.
| Armament | 1941 | 1944 |
| 46 cm (18.1 in) | 9 | 9 |
| 155 mm (6.1 in) | 12 | 6 |
| 127 mm (5 in) | 12 | 24 |
| 25 mm anti-aircraft | 24 | 162 |
| 13.2 mm anti-aircraft | 4 | 4 |
Nevertheless, Yamato was still sunken. They had too few AA guns. The first wave of attack consists of 280 planes and the second one over 100. It had taken 386 airplanes to bring down Yamato which only had 162 25 mm AA guns and a very small fleet of 9 escort ships. The special AA ammunitions for the 18.1" guns were found useless. Yes, they used the 18.1" guns as if they were shotguns.
The 25 mm AA guns, clustered around the bridge, were mostly destroyed by the bombs before Yamato's demise. Then the battleship had taken 10 torpedo hits (1 starboard, 9 port, the U.S. navy was very clever) and went off-balanced.
Since the Japanese Empire did not have the resources to build additional aircraft carriers and more airplanes in 1944, air support was out of the question. From a 20-20 hindsight point of view, how many 25 mm AA guns were required to defend against 386 U.S. airplanes? How many anti-torpedo weapons did it take? How many 18.1" turrets needed to go to make room for all these self-defense weapons? Did Yamato still have a chance if it cleans up the whole deck and give each of its sailor a 25 mm AA gun? -- Toytoy (talk) 09:58, 28 January 2009 (UTC)
- Japan did not have the proximity fuse at that time, using time fuses or barometric fuses as always, and nobody had radar-guided anti-aircraft guns on their ships. It's surprisingly hard to hit a moving airplane from a moving ship with a projectile by eye, and it's almost impossible to set a fuse so it goes off near an approaching plane. If the entire deck of the Yamato had been covered in AA guns, it would still have taken only one well-placed bomb to sink her. The battleship was a doomed dinosaur even before the Yamato was commissioned. (It was a beautiful ship, though, wasn't it?) --Milkbreath (talk) 12:37, 28 January 2009 (UTC)
- Also, if it was sufficiently well defended from air attack, it would have been attacked in other ways, such as a submarine torpedo attack or even a traditional battleship-to-battleship "crossing the T" engagement. High altitude bombers, which are out of range of anti-aircraft guns, would be another option, although their low accuracy means that many more bombs would need to be dropped. They also might not destroy the battleship directly, but could disable most of the anti-aircraft guns, allowing for low-altitude torpedo plane attacks. Mines could also be laid in it's path. If, by some miracle, the Yamato managed to survive all such attacks, the US might have even used an atomic bomb on it, when they became available, but, having only 2, they were in short supply. Still, if an "unsinkable" ship could be sunk instantly, especially if close enough to Japan to provide a sufficient demonstration, it might be a good use, perhaps sparing the 2nd city bombed, Nagasaki, of it's fate. Some of these approaches would have required a "strategic withdrawal" of the US fleet, to give time to prepare an adequate "reception". StuRat (talk) 14:51, 28 January 2009 (UTC)
- I don't know that nuking a battleship would have done much. The US didn't know at the time (and so it can't really be used to justify the theoretical decision), but Crossroads Able wasn't much of a ship-killer, and that sort of airburst is a good approximation of how an attack would have gone. — Lomn 18:25, 28 January 2009 (UTC)
- Incidentally, in reference to your topic heading, the idea that carrots significantly boost eyesight is a myth [3]. The myth was created intentionally by the British during WWII as a cover story to explain why the British were able to locate approaching German bombers so easily at night. The real answer is that the British had a top secret new invention: radar! Dragons flight (talk) 16:08, 28 January 2009 (UTC)
- Indeed. As I understand it carrots are only going to improve your night vision (day vision is completely unaffected) if you actually have a vitamin A deficiency (which cases nightblindness). If you have reasonable night vision already, it's not going to get any better. --Tango (talk) 18:11, 28 January 2009 (UTC)
- To take a different tack, I'll address the US Army Air Corps' approach to this problem. Faced with bombers that were outranging their fighter cover (and being mauled by the Luftwaffe for it), the AAC decided that the Flying Fortress, with its 10 or so antiaircraft guns, was insufficient to hold fighters at bay. They rebuilt B-17s into YB-40s, flying gunships that eschewed bomb loads in favor of additional powered turrets, ammunition, and armor. The effort, in short, flopped. YB-40s could not keep fighters at bay, could not keep up with the standard bomber formations, and could not drop bombs to salvage any cause for their inclusion in the flight line. It seems likely that the same sort of result would have come of a full conversion of a battleship to an antiaircraft platform -- as far as I know, no modern air assault has ever been substantially ablated by fixed-position fire (and yes, I'm lumping aerial and naval gunships into "fixed" -- relative to the attacking aircraft, they're effectively immobile), and a potentially effective battleship would have been lost in the process to boot. — Lomn 18:25, 28 January 2009 (UTC)
Without a radar-controlled AA system, you can hardly hit any airplane with no matter how many guns. And without a radar system capable of tracking and engaging dozens of targets at once (good computers + phase-array antennas), your defense can be saturated in a matter of seconds. But just how good were these WWII planes and their ammunitions? The steel hull of Yamato was very thick. It was designed to take serious punishments. Its sister ship Shinano was sunken by torpedos because of some poor design mistakes. If the design and manufacturing were perfect, could these outdated heavy weight battleships survive non-nuclear air attacks? Could a thick-shelled turtle return home? -- Toytoy (talk) 18:31, 28 January 2009 (UTC)
- Given that the Yamato and the Musashi were both sunk via aerial attacks, the answer is quite obviously "no". By 1944, US Naval bombers were lugging around one-ton bombs or torpedoes -- about the same throw weight as battleship shells. Since this wasn't an era where armor reigned supreme (contrast with the Battle of Hampton Roads for such a case]]), it's not controversial to say that any conventional ship* of the era could be sunk by any conventional aerial attack.
- *See Project Habakkuk for what could have been an interesting exception. — Lomn 21:18, 28 January 2009 (UTC)
Organ donor records[edit]
Who is the youngest organ donor? And who holds the record for being the oldest at the time of organ donation? In multiple organ donors, what is the maximum number of organs that have been donated by a person? Or is there any article similar to World records in medical science? Jay (talk) 10:03, 28 January 2009 (UTC)
- Youngest? Stillborn babies can become organ donors [4]. If you want to get technical, I guess you should count from conception. I'm somewhat doubtful anyone has records of that sort of thing Nil Einne (talk) 11:11, 28 January 2009 (UTC)
- Does destroying a few day old embryo in order to harvest embryonic stem cells count as "organ donation"? Technically, I suppose there weren't any "organs" yet. But in general, I believe finding the youngest organ donor is mostly a question of how one chooses to define "organ" and "donor". Dragons flight (talk) 16:01, 28 January 2009 (UTC)
- If you accept it, younger than this seems difficult! pma (talk) 22:30, 28 January 2009 (UTC)
- Making the queries more specific, I would like age to mean post live childbirth, and organ donation in the context of organ transplantation. Rather than the exact answers, what I'm looking for, in the absence of an available article, is ideas on which articles these details can be inserted into, or suggestions on title of a new article. Jay (talk) 08:36, 29 January 2009 (UTC)
- That sounds like a miscellaneous fact; in light of the objections raised above regarding ambiguous definitions, you might want to reconsider adding such information to any article. We have a trivia guideline information page; that page may help you decide the applicability of this policy. Nimur (talk) 18:40, 29 January 2009 (UTC)
- Making the queries more specific, I would like age to mean post live childbirth, and organ donation in the context of organ transplantation. Rather than the exact answers, what I'm looking for, in the absence of an available article, is ideas on which articles these details can be inserted into, or suggestions on title of a new article. Jay (talk) 08:36, 29 January 2009 (UTC)
- If you accept it, younger than this seems difficult! pma (talk) 22:30, 28 January 2009 (UTC)
- Does destroying a few day old embryo in order to harvest embryonic stem cells count as "organ donation"? Technically, I suppose there weren't any "organs" yet. But in general, I believe finding the youngest organ donor is mostly a question of how one chooses to define "organ" and "donor". Dragons flight (talk) 16:01, 28 January 2009 (UTC)
Osmole Confusion Part II[edit]
I asked a previous question (here) here, that was partially answered, but I find myself still confused.
The question was (edited a little): If I took 500 sodium ions and put them in 1 L in one container. In another contained, I put 500 glucose molecules. which has the greater osmolarity?
The answer that I found most helpful was this one, by Jayron32: So a 1 molar solution of NaCl will have double the osmolarity of a 1 molar solution of glucose, because dissolving 1 mole of NaCl in a liter of solution will produce double the number of particles that dissolving 1 mole of glucose will.
What I`m really confused about is the plasma osmolality equation. Posm=2xNA+GLUC/18+BUN/2.8 or something like that. Why does the concentration of sodium atoms contribute 36 times more to the osmolality than glucose?
Thanks! --Cacofonie (talk) 16:16, 28 January 2009 (UTC)
- The sodium does not contribute 36x more than the glucose -- you have to pay attention to the units. The short-hand calculation for plasma osmolality corrects for the different units that are given for the measurement of sodium (mEq/L), glucose (mg/dL), and BUN (mg/dL).
- The mass of glucose is ~180g/mol. Let's say you measure a typical plasma glucose of 90 mg/dL. First, let's convert to g/L for a plasma glucose of 0.9 g/L. Divide by 180g/mol and you get 0.005 mol/L or 5 mM. This is the same thing as dividing the plasma glucose by 18. A similar calculation is made for BUN. (corrected numbers... oops... stupid math... --- Medical geneticist (talk) 19:37, 28 January 2009 (UTC))
- Jayron already stated this, but again, in a biological context, sodium is never present on it's own but as a sodium salt, predominantly sodium chloride (but also, importantly, sodium bicarbonate). When in aqueous solution (as in blood), one mole of sodium chloride dissociates into a mole of sodium and a mole of chloride. Thus, in the calculation, you just double the amount of measured plasma sodium milliequivalents to account for the fact that the other (unmeasured) components (chloride and bicarbonate) of the sodium salts are present in solution.
- Adding together the molarity of sodium+chloride, glucose, and BUN gives a decent approximation of plasma osmolality.
- --- Medical geneticist (talk) 18:31, 28 January 2009 (UTC)
- It should also be noted that bicarbonate, being a weak electrolyte, introduces some serious complications should you want to be scrupulously correct in your calculations. Because bicarbonate, in a water solution, produces small amounts of SEVERAL different kinds of particles, it has a non-integer Van't Hoff factor, and as such, makes the calculations quite messy. However, as a first approximation, assuming sodium bicarbonate has a Van't Hoff factor of "approximately 2" usually gets things close enough for government work... Thus, as noted, you can assume "sodium" in this context to mean "sodium, and whatever negative ions it is draging along". So, we have some assumed NaX compound, which produces 2 moles of ions (Na+ and X-) and we just take it to mean that the identity of X (be it chloride, bicarbonate, or melange of several different kinds of ions) to be moot for colligative purposes. --Jayron32.talk.contribs 04:56, 29 January 2009 (UTC)
Wearing a coat inside making the cold outside feel colder.[edit]
I wear my winter jacket inside a lot of the time. I'm told that doing this will make the outside "colder" (it's winter) when I go outside. People recommend that I take off my coat when inside so that I don't get as cold outside.
Is this true? If so, how/why is this?
141.117.29.242 (talk) 16:40, 28 January 2009 (UTC)
- Perhaps it is because sweat glands, our natural cooling system, adjust to current conditions. When a coat is worn indoors, the glands open to produce more cooling. If the coat wearer then goes outdoors, it takes a little time for the sweat glands to adjust to the new condition. – GlowWorm. —Preceding unsigned comment added by 98.17.34.148 (talk) 16:58, 28 January 2009 (UTC)
- I agree with the above, in that if you're so hot you're sweating indoors, you should definitely take the coat off indoors. However, if you are cold enough to feel you need it indoors, then wear it. StuRat (talk) 17:04, 28 January 2009 (UTC)
- See Thermoregulation in humans. 76.97.245.5 (talk) 17:07, 28 January 2009 (UTC)
- That sounds about right, thanks a lot. 141.117.29.242 (talk) 17:17, 28 January 2009 (UTC)
- The article on thermoregulation says, "Horses and humans are two of the few animals capable of sweating." I wonder whether monkeys and apes sweat. It is also surprising that two creatures as unlike as horses and humans both sweat. Where did it start in the evolutionary chain? Did the same thing crop up twice in evolution? Also, sweating is one of those things that are a stumbling block to the theory of evolution - an organ cannot perform its function unless it is already perfected, or nearly perfected. – GlowWorm. —Preceding unsigned comment added by 98.17.34.148 (talk) 18:21, 28 January 2009 (UTC)
- Dogs have things that are essentially sweat glands in the their paws. This appears to be so that they can leave a scent trail for their pack-mates to follow rather than to cool themselves. That suggests an evolutionary means to adapt that kind of capability into something for cooling through relatively simple genetic changes. These "stumbling blocks to the theory of evolution" almost always turn out to be something like that. Some piece of biological machinery that was originally intended for one purpose gets re-purposed in some unexpected manner. The business about it only being in horses and humans sounds unlikely - but bear in mind that it's possible for the common ancestor of humans and horses to have had functioning sweat glands and that all of the other species descended from that point didn't need them anymore and lost the capability. Evolution is just as capable of deleting a feature as adding one. SteveBaker (talk) 18:48, 28 January 2009 (UTC)
- Or, it could be convergent evolution, although that seems even more unlikely. Interesting, sweat gland says that mammary glands are often considered modified sweat glands, which would suggest sweating pre-dates the evolution of mammals. --Tango (talk) 20:26, 28 January 2009 (UTC) --Tango (talk) 20:26, 28 January 2009 (UTC)
- Dogs have things that are essentially sweat glands in the their paws. This appears to be so that they can leave a scent trail for their pack-mates to follow rather than to cool themselves. That suggests an evolutionary means to adapt that kind of capability into something for cooling through relatively simple genetic changes. These "stumbling blocks to the theory of evolution" almost always turn out to be something like that. Some piece of biological machinery that was originally intended for one purpose gets re-purposed in some unexpected manner. The business about it only being in horses and humans sounds unlikely - but bear in mind that it's possible for the common ancestor of humans and horses to have had functioning sweat glands and that all of the other species descended from that point didn't need them anymore and lost the capability. Evolution is just as capable of deleting a feature as adding one. SteveBaker (talk) 18:48, 28 January 2009 (UTC)
- The use of sweating for thermoregulation is rare, but many organisms, including mammals, reptiles and insects, all excrete pheromones for various purposes, and it would not surprise me if scent glands developed for that purpose were modified to produce sweat glands. Dragons flight (talk) 20:42, 28 January 2009 (UTC)
- Sweat glands in dog's feet still had to be nearly perfected if they were to work at all. Sweat glands have to perform the complex function of converting bodily fluids, probably blood, into sweat. A duct to the surface also had to be developed. The same requrements apply to mammary glands. - GlowWorm —Preceding unsigned comment added by 98.17.34.148 (talk) 19:31, 28 January 2009 (UTC)
- Sweat could have started off as just blood plasma or interstitial fluid and still served a purpose, so there it doesn't have to be one big leap. --Tango (talk) 20:26, 28 January 2009 (UTC)
- I still think the organ-specialism problem presents a serious difficulty to the theory of evolution. Those who wish to deny this difficulty say, in any particular case, that there was adaptation of something else. But they cannot show a partially-adapted organ (midway between two functions) in living things or in fossils. Their denial of the difficulty seems to me to be in the same category as the original denial of evolution itself. There was immense opposition to the theory - in England especially - when Darwin's work was first published. However, by now a great mass of supporting evidence for the theory of evolution has been adduced. There is so much supporting evidence that the main thrust of the theory cannot be denied except by religious fundamentalists, who take a nonscientific approach. But I think there is more to be said - in a scientific manner - about evolution. And it is something important. – GlowWorm
- One major part of the problem may just be that soft tissue doesn't fossilise particularly well so it is very difficult to tell when organs an extinct creature had. Even if these missing links exist, we would probably struggle to find them. --Tango (talk) 22:47, 28 January 2009 (UTC)
- Organs are never "partially adapted". To suggest such a thing implies a teleology to evolution — as if an organ starts out performing function A, but then somehow decides that it should instead be performing function B and starts moving that way. The only way organs can be repurposed is if, at every single step along the way, the changes provide a larger advantage (in performing function B better) than they do disadvantage (in performing function A worse). Every organism in the evolutionary chain that leads to us was a successful reproducer in its own right, there are no inferior halfway points between well-adapted creatures. Maelin (Talk | Contribs) 00:50, 29 January 2009 (UTC)
- That's not entirely true - as long as there isn't a significant disadvantage to the change it may spread just by random chance, especially in a small population. Advantageous mutations are more likely to spread, but that doesn't mean that all neutral mutations die out. --Tango (talk) 14:00, 29 January 2009 (UTC)
- I still think the organ-specialism problem presents a serious difficulty to the theory of evolution. Those who wish to deny this difficulty say, in any particular case, that there was adaptation of something else. But they cannot show a partially-adapted organ (midway between two functions) in living things or in fossils. Their denial of the difficulty seems to me to be in the same category as the original denial of evolution itself. There was immense opposition to the theory - in England especially - when Darwin's work was first published. However, by now a great mass of supporting evidence for the theory of evolution has been adduced. There is so much supporting evidence that the main thrust of the theory cannot be denied except by religious fundamentalists, who take a nonscientific approach. But I think there is more to be said - in a scientific manner - about evolution. And it is something important. – GlowWorm
- Another simpler explination is that if you wear your jacket indoors you will sweat in it (not necessarially because you are hot but because you normally sweat a little in most of your normal daily activities). When you go back outside this slight dampness will make you significantly colder. It is the same effect if you've ever worn the same socks two days in a row and notice that on the second day your feet are colder. This is also why they advice changing cloths before bed when doing winter camping, to make sure the cloths are dry and clean to keep you as warm as possible. Anythingapplied (talk) 20:36, 28 January 2009 (UTC)
Not just sweat; we have a variety of ways to regulate our temperature, such as dilating blood vessels. Someone who has adapted to live in Scotland is likely to feel rather hot in Miami, and someone adapted to Miami is probably going to feel chilly in Scotland. Even if the two people wear the same clothes in the same places. We adapt to regulate our temperatures so our internal body temperature stays constant. On a smaller scale, if you wear more clothes than strictly necessary inside in the winter you'll get used to being snuggly and warm while regulating your temperature for warmer conditions. When you go outside, you need to shift to working harder at keeping warm. In particular, if you wear your coat inside you're likely to have more warm blood flowing near the surface of your skin. When you go outside you're suddenly in a situation where your skin is much warmer than the outside air (far more so than if you hadn't kept your coat on), so you quickly lose a lot of heat. Losing heat feels cold. 79.66.105.133 (talk) 20:35, 29 January 2009 (UTC)
Synthesis of Benzene[edit]
Hi, I was told you cannot "make" benzene from cyclohexane as Benzene cannot be treated as a molecule with 3 isolated double bonds- as bond lengths indicate equal bond lengths.
So is the following not possible then? Because you can Birch reduce Benzene to cyclohexane.
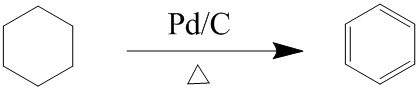
Notify me of a response by copying and pasting the following on my talk page, Thanks --DFS454 (talk) 17:12, 28 January 2009 (UTC)
{{newmessages|Wikipedia:Reference desk/Science#Synthesis of Benzene}}
- Birch reduction of benzene gives cyclohexadiene, not cyclohexane, and dissolving-metal reductions don't usually touch simple alkenes at all. That's two bits of evidence that benzene is something other than "cyclohexatriene". DMacks (talk) 18:36, 28 January 2009 (UTC)
- The alternating-double-bond model that makes benzene appear to be "cyclohexatriene" is largely an artifact of the diagrams used to draw benzene, or reliance upon models of molecular bonding which fall short of fully explaining the situation. There are several good models of bonding which actually reliably predict that benzene will NOT behave as "cyclohexatriene" would be expected to. For example, hybridization theory shows that every carbon atom is "sp2" hybridized, and its just not possible to justify having 6 identical atoms bonded to each other with alternatingly different length and strength bonds. It just doesn't make sense. Likewise, Molecular Orbital Theory, sort of a "hybridization theory on steroids", makes a perfect prediction of the "correct" structure of benzene. The problem is that the standard "stick and ball" model, or the condensed structural formula model shown above, do not allow themselves to easily represent the reality of the delocalized pi-bonding system present in benzene. Its a case of inadequate models more than anything. Once you realize what benzene really is, as explained by hybridization theory and MOT, then its properties and reactions make MUCH more sense. --Jayron32.talk.contribs 04:48, 29 January 2009 (UTC)
- Such excellent answers have rarely benzene at the Ref Desk. StuRat (talk) 14:56, 29 January 2009 (UTC)
- Yeah, without MO theory, it's hard to explain why benzene is like this, but cyclooctatetraene is really just cyclooctatetraene alternating single and double bonds. DMacks (talk) 18:22, 29 January 2009 (UTC)
- One of the issues would be the comformation of the cyclohexane. It has a chair or boat conformation while the benzene ring is flat. - Mgm|(talk) 08:52, 30 January 2009 (UTC)
While reading, with some amusement, about the cello scrotum hoax, I noticed the original letter to the editor finishes, "... — I am, etc. <signed>" It appears, from the other letters on that page and Ghits, [5] that this is a standard sign off for letters published in this journal. What does the etc replace and why is it used by all correspondents? Rockpocket 18:10, 28 January 2009 (UTC)
- I'm guessing - but I get the impression that somewhen in (roughly) Victorian times, there was a large amount of flowery crap you had to put on the end of a letter ("I am yours faithfully") with the complexity of the protocol required to say 'faithfully' on business letters unless it's to a loved one ('eternally') or to a lawyer ('respectfully') or a government official or some minor royalty ('loyally'). Keeping all of that straight - especially when writing (in effect) to a large number of unknown people in a journal - got so bothersome that people would just stick an 'etc' in there an let people figure it out for themselves! It's come a long way from there to ~~~~ or '-- Steve' which is what I use on email! SteveBaker (talk) 18:36, 28 January 2009 (UTC)
- Yes, you are correct. I just looked at some of the letters from the first issues in the 1840s and, back then the standard sign-off was "... — I am, gentlemen, your very obedient servant,<signed>." The "gentlemen" are the editors of the journal to whom the letters are addressed. Rockpocket 19:11, 28 January 2009 (UTC)
- Note, "yours" in "yours faithfully",etc., is short for "your servant" (as in "I'm at your service", not necessary actually employed to scrub floors!). We do, of course, have an article: Valediction. --Tango (talk) 19:35, 28 January 2009 (UTC)
- Which somehow fails to mention the Italian form sono vostro schiavo, "I am your slave"; a flowery formula of the highborn that somehow morphed into today's extremely informal ciao. --Trovatore (talk) 23:26, 28 January 2009 (UTC)
- Wikipedia - the free encyclopedia that anyone can edit. SteveBaker (talk) 00:29, 29 January 2009 (UTC)
- Which somehow fails to mention the Italian form sono vostro schiavo, "I am your slave"; a flowery formula of the highborn that somehow morphed into today's extremely informal ciao. --Trovatore (talk) 23:26, 28 January 2009 (UTC)
- Note, "yours" in "yours faithfully",etc., is short for "your servant" (as in "I'm at your service", not necessary actually employed to scrub floors!). We do, of course, have an article: Valediction. --Tango (talk) 19:35, 28 January 2009 (UTC)
Isn't the "etc." put in by the editors? the original letter would still have a valediction.124.176.236.32 (talk) 07:41, 29 January 2009 (UTC)
- Such letters to the editor are always published in exactly the same format, so I would expect people to just write them in that format to start with. If they don't, it might well be edited to fit the standard format (there is usually a disclaimer somewhere saying they might edit your letters). --Tango (talk) 13:49, 29 January 2009 (UTC)
- Hmmm - that might make sense. If the author wrote TO the editor using a valediction appropriate to the editor - then the editor might wish to redact it when printing the letter to be read by others to whom that valediction was not appropriate. This stuff mattered a lot more 35 years ago than it does today. I'm fairly sure we'd have been taught the correct way to address a letter to a person of importance when I was in high school around about then...sadly, that's exactly the kind of thing I forgot about a nanosecond later! SteveBaker (talk) 18:59, 29 January 2009 (UTC)
- I don't think it's a matter of appropriateness, they're just abbreviating it because it's very long. These days "Yours faithfully" would be standard (since letters to the editor are usually addressed "Dear Sir"), but publications like the BMJ have been around so long they probably like to stick to old fashioned versions just for the hell of it. --Tango (talk) 20:08, 29 January 2009 (UTC)
- Hmmm - that might make sense. If the author wrote TO the editor using a valediction appropriate to the editor - then the editor might wish to redact it when printing the letter to be read by others to whom that valediction was not appropriate. This stuff mattered a lot more 35 years ago than it does today. I'm fairly sure we'd have been taught the correct way to address a letter to a person of importance when I was in high school around about then...sadly, that's exactly the kind of thing I forgot about a nanosecond later! SteveBaker (talk) 18:59, 29 January 2009 (UTC)
Autodidact: how to prove that you learned something[edit]
How can an autodidact prove that he learned something about this or that science? Is there any independent examination out there?--80.58.205.37 (talk) 18:16, 28 January 2009 (UTC)
- Well, you could try answering 10 questions a day on the WP RefDesk and if you get through a month without getting ripped to shreds by the other editors then you've probably made it! But seriously: I don't know at what level you are working - but you could take a look at the resources available to parents of home-schooled children...somehow they must be able to do it. SteveBaker (talk) 18:39, 28 January 2009 (UTC)
- Actually, if you answer enough Q's here you will get ripped to shreds, even if the answers are correct, if they lack references to expert sources (even though they agree with the experts). If, on the other hand, your answers are correct, but disagree with the current experts, then God help you. StuRat (talk) 14:51, 29 January 2009 (UTC)
- In the same line: General Educational Development certifies that the taker has American or Canadian high school-level academic skills. For more advanced skills you could try the GRE. It is done before graduate school, however, I don't think you have to have a Bachelor degree to apply for it. There are also several IT certificates that don't require that you follow an specific path. Mr.K. (talk) 18:58, 28 January 2009 (UTC)
- You could make and publish ground-breaking discoveries. --NorwegianBlue talk 19:36, 28 January 2009 (UTC)
For jobs at my organization, experience can be substituted for formal education. The idea being that if you have done a type of work sucessfully for a long time, that is as good as or better that a degree saying that you know how to do the job. ike9898 (talk) 19:48, 28 January 2009 (UTC)
I think we would be better able to address your question if we know: (1) the (equivalent) education level; (2) area of study; and, (3) country you are interested in, since there is a huge difference between being a "certified" autodidact in, say, web-design vs surgery. Abecedare (talk) 20:08, 28 January 2009 (UTC)
- No, I don't have any interest in becoming a certified surgeon or certifying my knowledge in another highly regulated profession. I only have an interest in certifying my knowledge in some natural sciences above undergraduate level without putting a huge amount of money and time into a degree program. So far I found Graduate_Record_Examination#GRE_Subject_Tests. --88.0.97.125 (talk) 10:43, 29 January 2009 (UTC)
- edit conflict
- The GRE Physics subject test is a good start, but even some physicists consider it "irrelevant" because it emphasizes certain areas of research over others. (If only I had known ahead of time!) This is a general problem of any testing methodology.
- Whether you are an autodidact or a formally trained scientist, you are fundamentally trying to establish credibility, if not expertise, in a specific subject area. The best way to do this is to participate in mainstream discussions with your specific community of interest. In modern science contexts, such conversations take the more structured format of journal publications, conference presentations, and grant requests. In the (slightly less formal) Wikipedia community, you can follow SteveBaker's advice - the Reference Desk is a cut-throat community of hardened misanthropic science-enthusiasts who will gladly correct your errors until you learn the ways of its in-crowd. It's not so very different from regular academia. Nimur (talk) 18:45, 29 January 2009 (UTC)
