User:Tekmeme/2-Methylbutanal
| This is not a Wikipedia article: It is an individual user's work-in-progress page, and may be incomplete and/or unreliable. For guidance on developing this draft, see Wikipedia:So you made a userspace draft. Find sources: Google (books · news · scholar · free images · WP refs) · FENS · JSTOR · TWL |
2-Methylbutanal new article content ...
| |

| |

| |
| Names | |
|---|---|
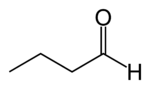
| IUPAC name
2-methylbutanal
| |
| Systematic IUPAC name
2-methylbutanal | |
| Other names
Butanal, 2-methyl-; Butyraldehyde, 2-methyl-; 2-Formylbutane; 2-Methylbutyric aldehyde; 2-Methyl-1-butanal; Methylethylacetaldehyde; 2-Ethylpropanal; Acetaldehyde, ethylmethyl-
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChemSpider | |
| KEGG | |
PubChem CID
|
|
| UNII | |
| |
| |
| Properties | |
| C5H10O | |
| Molar mass | 86.1323 g/mol <<< |
| Density | 0.806 g/ml |
| Melting point | −79 °C |
| Boiling point | 94-96 °C |
| 1.1 g/100 mL (25 °C) | |
| Hazards | |
| NFPA 704 (fire diamond) | |
| Flash point | 4 °C |
| Explosive limits | 2.5–12.5% |
| Related compounds | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Tracking categories (test):
References[edit]
- ^ Merck Index, 11th Edition, 1591
External links[edit]


