User:Sennalen/sandbox/COVID
| This is not a Wikipedia article: It is an individual user's work-in-progress page, and may be incomplete and/or unreliable. For guidance on developing this draft, see Wikipedia:So you made a userspace draft. Find sources: Google (books · news · scholar · free images · WP refs) · FENS · JSTOR · TWL |
COVID-19 zoonosis theories are scientific hypotheses proposing that SARS-CoV-2, the causative agent of COVID-19, was first introduced to humans through zoonosis (transmission of a pathogen to a human from an animal). A zoonotic spillover event is the possible origin of COVID-19 that is considered most plausible by the scientific community.[a] It is unknown where SARS-CoV-2 originated or how it first infected humans.[b] Human coronaviruses including SARS-CoV-2 are suspected to be zoonotic diseases originally acquired through spillover infection from animals.[3] The original animal host or reservoir of SARS-CoV-2 is unknown.[c] The question of whether humans acquired infections directly from a natural reservoir species or through an intermediate host is unresolved.[3]
Putative origin in bats[edit]
SARS-CoV-2 is believed to originate in bats.[5][6] SARS-CoV-2 is a coronavirus in genus Betacoronavirus, subgenus Sarbecovirus, alongside SARS-CoV-1 and many other bat-related coronaviruses.[7] There is a consensus that bats are the primary natural reservoir for human and animal coronaviruses.[8] Based on serological and molecular studies, Chinese horseshoe bats were the putative reservoir for SARS-CoV-1.[9] Bats were also a putative reservoir for the related betacoronavirus MERS-CoV, though the evidence for this is less conclusive than the role of camels as a reservoir for MERS.[10] Several bat species have special cellular mechanisms to resist proinflammatory cytokines associated with Betacoronavirus virulence.[11] Guo et al. wrote that spike proteins in SARS-related coronaviruses coevolved with bat ACE2 receptors in an evolutionary arms race.[12] Coronaviruses circulate among several species of bat, which may share caves with one another.[13] Humans visiting caves to collect guano or for other reasons may risk exposure to bat-borne viruses.[14] There is no evidence that a Sarbecovirus has been directly transmitted to a human from a bat.[6]

Bats, along with their viruses, have large overlapping geographic ranges in Southeast Asia.[15] There is a great concentration and diversity of bat-related coronaviruses in Southern and Southwest China.[11] A 2021 survey of coronaviruses in bats in China found no close relatives of SARS-CoV-2 in circulation.[16] The most similar known viruses to SARS-CoV-2 include bat coronaviruses BANAL-52 with 96.8% nucleotide sequence identity,[17] RaTG13 with 96.1% identity,[2] RpYN06 with 94.5% identity,[11] and RmYN02 with 93% identity[18] RaTG13 was not the direct progenitor of SARS-CoV-2.[19] Temmam et al. found no serological evidence for exposure to BANAL-52 among bat handlers and guano collectors in the area of Laos where it was sampled.[20] Lytras et al. wrote that "SARS-CoV-2 can be unambiguously traced to horseshoe bats".[21] They estimated SARS-CoV-2's most recent common ancestors with RmYN02 and RaTG13 to have diverged 40 and 50 years ago, respectively.[22] Simiarly, they estimated common ancestors with viruses sampled from Cambodia and Thailand dated between 1841 and 1938. Deng et al. wrote, based on bioinformatic analyses, that SARS-CoV-2 evolved in the same host environment as other bat coronaviruses including RaTG13.[23]
Novel features of SARS-CoV-2[edit]
The receptor binding domain of the SARS-CoV-2 spike protein has an insertion of amino acids between its S1 and S2 subunits.[24] This insertion affects its interaction with human ACE2 enzyme, with significant implications for its infectivity.[18][2] Among Sarbecoviruses, only SARS-CoV-2 and RmYN02 have such an insertion, suggesting differences in reservoir species, intermediate hosts, or evolutionary pathway.[18] SARS-CoV-2's close relative RaTG13 has low similarity in the receptor binding domain and does not have affinity for human ACE2 receptors.[2] The receptor binding motif is the portion of SARS-CoV-2 that is most diverged from RaTG13.[25] The SARS-CoV-2 receptor binding domain is more similar to those of pangolin coronaviruses.[18] Viruses including BANAL-52 isolated from bats in Laos showed high similarity to the SARS-CoV-2 receptor binding domain in amino acid residues but less than 76.4% nucleotide identity across the spike protein.[3]
SARS-CoV-2 is unique among its known relatives in bats in its possession of a functional furin cleavage site.[2] It is the only known member of Sarbecovirus with such a site.[26] BANAL-52 and related sequences that are generally close to SARS-CoV-2 in the receptor binding domain do not contain a furin cleavage site.[27] Within genus Betacoronavirus, furin cleavage sites are common in subgenera Merbecovirus and Embecovirus.[26] Wu and Zhao wrote that furin cleavage sites have independently evolved six times in different clades of Betacoronavirus.[26] Furin cleavage sites also evolved in Alphacoronaviruses and Gammacoronaviruses independently of Betacoronavirus.[26]
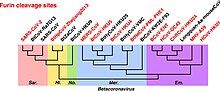
Furin cleavage contributes strongly to the transmissibility and pathogenicity of SARS-CoV-2 in humans.[2][28] SARS-CoV-2 variants lacking the furin cleavage site are transmissible between humans, but much less effectively.[29] The furin cleavage site is sometimes described as "polybasic" on account of its particular motif of basic amino acids.[28] SARS-CoV-2 shares amino acid identity with a furin cleavage site of human ENaC α subunit.[30] [31][d] Human ENaC is identical only to that of a few great apes and Pipistrellus kuhli.[32]
The furin cleavage site could hypothetically have arisen in several ways: recombination between an ancestor of SARS-CoV-2 and a virus already possessing a furin cleavage site, passaging in an intermediate host, or adaptation by an asymptomatic early human infection.[33]
SARS-CoV-2 is also distinct among human coronaviruses for having a single intact ORF8 gene rather than "a" and "b" subunits.[1]
[edit]
Previous emergence of SARS-CoV-1 and MERS-CoV showed that Betacoronaviruses represent a risk for emergence of diseases threatening to humans.[34] Understanding the reservoirs and intermediate hosts of coronaviruses can aid in prediction of outbreaks.[35] Increased awareness of coronaviruses due to the 2002–2004 SARS outbreak motivated research into "their persistence in reservoir hosts, the molecular mechanisms governing their emergence and pathogenesis in the human population, and the factors required for successful vaccine and therapeutic interventions."[36] Bats are known to harbor persistent populations of coronaviruses, and under conditions of persistent infection, coronaviruses tend to accumulate mutations that allow their receptor binding domains to interact with cross-species orthologs of the target receptors.[37] By 2010, in vitro experiments had confirmed that modifications to the spike protein receptor binding domain could enable human infection by several SARS-related coronaviruses.[38] Graham and Baric at that time wrote, "that the question of emergence of another pathogenic human coronavirus from bat reservoirs might be more appropriately expressed as 'when' than as 'if'."[39]
Since the SARS1 outbreak, there have been several national and international efforts to search for unknown viruses with zoonotic potential in Asia and Africa. Wuhan, China became a center of excellence for coronavirus research. By 2019, research with coronavirus samples was conducted by eight institutions at nine sites in Wuhan.[40] Starting in 2004, over 15,000 field samples from South Asia and Southeast China were collected in Wuhan. From these, about 220 SARS-like viruses were identified, of which about 100 have been published.[41] In 2013, researchers identified for the first time a virus (designated WIV1) infecting bats that was capable of using human ACE2 for cell entry without further adaptation in its receptor binding domain.[42] To study its infectivity, Menachery et al. created a chimeric virus incorporating the WIV1 spike protein into a backbone of mouse-adapted SARS-CoV-1.[43] They confirmed its ability to infect human airway epithelial cells in culture. It also infected humanized mice in vivo but was rapidly cleared by the immune system. Menachery et al. concluded that the WIV1 clade represented a threat for future human pathogen emergence, but it would require additional adaptations to be capable of epidemic spread.[44]
Beginning in 2011, Chulalongkorn University in Thailand and U.S. government sponsors began collecting field samples with the goal of discovering previously unknown pathogens. In 2021, citing risks of collecting unknown viruses and a lack of useful data obtained, supervising physician Thiravat Hemachudha called for an end to collection and for the destruction of samples.[45]
In September 2019, Li et al. reported detection of antibodies against SARS-like coronaviruses in volunteers from rural Southwest China as possible evidence of spillover.[46]
From 2008 through the present, a number of researchers have created chimera involving coronavirus spike proteins.[47] Menachery et al., writing in 2016, highlighted the potential of chimeric viruses to contribute to research on new therapeutics, but cautioned that such experiements had the potential to accidentally create new viable human pathogens.[44] In a 2018 proposal to DARPA, EcoHealth Alliance in cooperation with the Wuhan Institute of Virology proposed to search for SARS-like viruses possessing furin cleavage sites, and in the case that none were found, to synthesize one for study.[48] DARPA did not provide funding, but it is unknown if the work was carried out.[31] Against this background, some scientists have hypothesized that the origins of SARS-CoV-2 involved a research-related incident, an accidental release either of a sample collected from the wild or a synthesized reagent.[49][31] There is no evidence that a natural or artificial direct progenitor of SARS-CoV-2 was at the Wuhan Institute of Virology before the pandemic.[50] Holmes et al. wrote that while the possibility of a laboratory origin "can not be entirely dismissed", it is "highly unlikely" compared to natural origins.[51] Wu and Zhao wrote that the multiple independent evolutions of furin cleavage sites in various clades of Coronavirus demonstrates that a natural origin is possible.[26] Balloux et al. wrote that natural emergence through recombination is the parsimonious explanation.[1]
Processes of host adaptation[edit]
The early history of SARS-CoV-2 adaptation to humans is unknown[52] SARS-CoV-2 has an expanded host range compared to SARS-CoV-1 and MERS-CoV.[53][54] Li et al. wrote that SARS-CoV-2 (along with SARS-CoV-1 and MERS-CoV) are generalist viruses, not specifically adapted to humans, meaning they have potential to spill over to many species and establish new natural reservoirs after adaptive evolutionary changes.[55] Tai et al. wrote that it was unlikely that an ancestor of SARS-CoV-2 could establish human infections without undergoing adaptations.[52] SARS-CoV-2 has been found to have poor ability to infect bat cells.[2]
Within a single host, a variety of single-nucleotide variations arise through random mutations and genetic drift giving rise to viral quasispecies.[56] SARS-CoV-2 mutates at a slower rate than is typical of RNA viruses.[56] The main host-derived driver of mutation is editing by APOBEC proteins.[56] Negative selection by host immune processes causes convergent evolution towards immune escape.[56] Persistence of infection is correlated with quasispecies diversity, but the direction of causality for this is unknown.[56]
Actual host susceptibility can differ significantly from in silico predictions.[55][57] The process of host adaptation has been studied in humanized mice as well as by generating mouse-adapted viral strains through serial passage.[58] Wild-type mice are only weakly succeptible to the original SARS-CoV-2 strain.[58]
Recombination[edit]
Compared to other single-stranded RNA viruses, coronaviruses have increased tendency to undergo genetic recombination, which allows them to exchange genetic material with close relatives co-infecting the same organism.[59] The origin of SARS-CoV-1 is believed to involve multiple recombination events.[60] Recombination between strains of SARS-CoV-1 is common.[61] Recombination between various SARS-CoV-2 variants of concern has been reported.[62]

Temmam et al. identified 14 recombination breakpoints in the evolution of sarbecoviruses.[63] They wrote that SARS-CoV-2 is a mosaic of five or more ancestor viruses.[17] Temmam et al. called a recombination breakpoint seven nucleotides upstream of the receptor binding domain region of S1.[17] Qiang et al. wrote that inferred phylogenies of SARS2-related coronaviruses might be explained by recombination.[64] Lytras et al. identified the SARS-CoV-2 spike open reading frame as a recombination hotspot.[65] They speculate that the SARS-CoV-2 genome may involve repeated recombination events overprinting regions that were already themselves products of recombination.[66] Temmam et al. wrote that due to the limited diffusion of bat viruses in mammals, co-infection required for recombination in mammals was unlikely.[67] Therefore, they considered it more likely that the furin cleavage site arose in a bat reservoir prior to spillover.
Selection[edit]
After cross-species transmission of a virus, rapid evolution and positive selection are expected.[68] Several studies found only weak signs of adaptive evolution early in the COVID-19 pandemic.[e] Kang et al. wrote that SARS-CoV-2 had exhibited relatively little genetic variation by 2021.[68] Tai et al. wrote that population expansion rather than positive selection explained the mutation frequency spectrum during the early pandemic.[70] Cagliani et al. wrote that the SARS-CoV-2 genome overall shows evidence of "strong to moderate" purifying selection.[71] Accessory open reading frames, especially ORF8, showed weak to neutral selection. The general lack of positive selection during the early outbreak of SARS-CoV-2 contrasted with the evolutionary course of SARS-CoV-1.[72]
Strong evidence of positive selection was found however in the spike protein S1 subunit, which contains the receptor binding domain.[25][73] Cagliani et al. also found weak positive selection for nsp1.[74]
Genome instability in the spike protein is typical of coronaviruses in general, favoring the production of numerous spike protein variants.[62] The receptor binding domain is a significant factor in host tropism, or the variety of species a virus can infect.[75][76] Coronavirus adaptation to a new host often requires mutations in the receptor binding domain.[77] The ability of the SARS-CoV-2 receptor binding domain to interact with the ACE2 receptor is a major determinant of its host range.[2] Kang et al. identified a single nucleotide polymorphism relative to RaTG13 in the spike protein, consistent among all of more than 180,000 SARS-CoV-2 samples, affecting glycosylation of the receptor binding domain.[78] Using a reverse genetics system to generate an ancestral-like mutant, they confirmed that the putative ancestral form of this SNP was much less transmissible in human cells.[79]
Early in the pandemic, SARS-CoV-2 diverged into two lineages differing from one another in two positions with strong genetic linkage.[80] This leads to either a leucine or serine in a particular position, causing the lineages to be named "L" and "S". They have also been called "A" and "B", with "A" corresponding with "S" and "B" with "L". Qiang et al. write that the persistence of the linkage between the two sites suggests epistasis, though the mechanism is unknown.[81] The adaptations required for a functional furin cleavage site also come at a cost to the protein's structural stability.[82] Pathogenic mutations are therefore accompanied by simultaneous epistatic mutations compensating for this instability. Consequently, the furin cleavage site is quickly lost to random mutations in cell culture. Different solutions to this stability problem underlie the divergence of lineages A and B. It is also integral to the emergence of variants of concern.
Host editing[edit]
Besides ACE2, Li et al. identified 590 mammalian host genes whose transcription levels correlated with SARS-CoV-2 cross-species transduction rates, the most significant of those factors being zinc finger protein.[83] From December 2019 through February 2020, SARS-CoV-2 mutations showed a bias towards converting cytosine to thymine.[84] Tai et al. wrote that this may have been due to a CpG targeting mechanism involving zinc finger antiviral protein.[85] In contrast, the divergence of SARS-CoV-2 and RaTG13 from ancestral sarbecoviruses involved the opposite bias.[85] Qiang et al. wrote that editing by APOBECs could account for the codon bias.[86] MacLean et al. wrote that the codon bias is evidence that a common progenitor of SARS-CoV-2 and RmYN02 underwent positive selection in bats without circulation in other species.[87] The S/A lineage is more similar to presumed ancestral sequences in its two nucleotide variations.[88] Pekar et al. wrote that lineage L/B was the earlier strain in humans, and that lineage S/A reverted to ancestral nucleotides due to mutational codon bias.[89]
Host proteases[edit]
The majority of sarbecoviruses are not dependent on ACE2 for cell entry.[90] Guo et al. divide the sarbecoviruses into four clades, the first being ACE2-using respiratory viruses including SARS-CoV-1, SARS-CoV-2, and WIV1. Relative to clade 1, clades 3 and 4 have a one residue deletion in the receptor binding domain and diminished ability to use ACE2. Clade 2 has two deletions and does not interact with ACE2. Clades 2 through 4 are more difficult to isolate or propagate in cell culture, and consequently have been less studied. ACE2-independent infection by sarbecoviruses depends on high levels of trypsin, a digestive protein, in the host environment. Trypsin may compensate for other missing or compatible host proteases. The ubiquity of furin compared to tripsin would allow the gain of a furin cleavage site to expand viral tissue tropism.[91] Guo et al. identified clade 1 and 2 sarbecoviruses in Rhinolophus fecal samples, suggesting that both types naturally replicate in the bat digestive tract.[90] Bats with SARS-like infections in the wild showed no viral replication in respiratory droplets.[92] A fecal-oral transmission is an alternative route for some respiratory viruses.[90] No higher incidence of Sarbecoviruses has been reported in workers who come into direct contact with guano.[6] Temmam et al. found that BANAL-236, a SARS-CoV-2-related virus isolated from bats in Laos, acts as an enteric virus in macaques.[93] Six passages of BANAL-236 in humanized mice did not result in lung tropism, ACE2 affinity, or a furin cleavage site.[94]
Timing of spillover[edit]
The first case of COVID-19 documented by Chinese authorities was identified on December 8, 2019, in Wuhan, China,[41] after symptoms said to have started on December 1.[95] There are unconfirmed reports of cases in November 2019.[96] There is some evidence that SARS-CoV-2 was present in Italy in December 2019.[96][97][98] Kumar et al. wrote that the first samples collected from patients in Wuhan were not the direct progenitors of all SARS-CoV-2 infections globally.[98] They estimated that the most recent common ancestor of samples collected globally early in the pandemic arose in October 2019. The index case, or incident of zoonosis, could have been earlier still.
To explain limited evidence of positive selection, Tai et al. propose that SARS-CoV-2 progenitors circulated cryptically among humans before the pandemic, gradually accumulating adaptive mutations.[52] Temmam et al. wrote that if there was pre-pandemic circulation, it was likely in the form of an enteric virus.[67] Balloux et al. wrote that the limited diversity of viral sequences by January 2020 suggested a single, recent introduction event.[97] Pekar et al. wrote that based on epidemiological simulations, the most recent common ancestor of circulating human strains was in a non-human animal prior to spillover.[99][f] They estimated lineage B/L spilled over to humans between October 23 and December 8, and that lineage A/S separately spilled over between October 29 and December 14. Temmam et al. wrote that a double spillover from a mammalian intermediate host was "difficult to reconcile" with the limited transmissibility of unadapted strains and remote prior probability of a furin cleavage site arising spontaneously.[67]
In contrast to SARS-CoV-1, the initial outbreak of SARS-CoV-2 was reported in only one city.[100] Chinese epidemiological surveillance did not report any other pneumonia outbreaks in autumn 2019.[101] Graham and Baric wrote that in the case of SARS-CoV-1, virus populations circulated and adapted to civets and humans over the course of two years before the recognized outbreak.[92]
Reverse zoonosis[edit]
Transmission of SARS-CoV-2 from humans to animals, known as reverse zoonosis or anthroponosis, is possible.[102] Reverse zoonotic or experimental infection with SARS-CoV-2 has been reported in 31 animal species.[103] It is not believed that wildlife plays a significant role in the ongoing circulation of SARS-CoV-2 among humans.[104]

SARS-CoV-2 variants adapted to mink were found co-circulating among humans and farmed mink.[3] Mink are the only animal in Europe or North America to experience widespread SARS-CoV-2 outbreaks. Transmission from human to mink has occurred multiple times, in most cases not resulting in a sustained mink outbreak.[72] Strong evidence was seen of positive selection in mink after spillover, concentrated in the receptor binding motif.[105]
Transmission back to humans has been documented for mink, hamsters, and cats.[104] Transmission to humans from deer is suspected.[104] Transmission of SARS-CoV-2 among domestic cats has been confirmed.[106]
Li et al. wrote that SARS-CoV-2 may be less able than SARS-CoV-1 to pass from humans to animals.[76]
Hypothesized intermediate hosts[edit]
In the outbreak of SARS-CoV-1, palm civets, raccoon dogs, ferret badgers, red foxes, domestic cats, and rice field rats were possible vectors.[9] Graham and Baric wrote that human and civet infections likely stemmed from an unknown common progenitor.[107] Patrick Berche wrote that the emergences of SARS-CoV-1 and MERS-CoV appeared to be sequential processes involving intermediate hosts, co-infections, and recombination.[108] In contrast with the rapid identification of animal hosts for SARS-CoV-1 and MERS-CoV, no direct animal source for SARS-CoV-2 has been found.[23] Holmes et al. wrote that the lack of intermediate host is likely because the right animal has not been tested so far.[19] Frutos et al. proposed that rather than a discrete spillover event, SARS-CoV-2 arose in accordance with a circulation model, involving repeated horizontal transfer among humans, bats, and other mammals without establishing significant reservoirs in any of them until the pandemic.[109]

Pangolins have been considered a possible reservoir of SARS-CoV-2.[110] Pangolins are sometimes sold in wet markets in China, where they are considered a culinary delicacy and a component of traditional medicine.[6] The highest sequence similarity to the SARS-CoV-2 spike receptor binding domain was found in a coronavirus infecting Sunda pangolins in Guangdong province.[53] Pangolins are frequently smuggled to China.[66] These viruses were first discovered in the course of anti-smuggling operations.[16] Lytras et al. wrote that, consistent with a lack of reported infections of pangolins in Malaysia, they were likely infected after being trafficked into China.[66]
The SARS-CoV-2 receptor binding domain shares more synonymous substitutions with a pangolin-CoV than RaTG13.[111] The binding potential of SARS-CoV-2 to pangolin ACE2 however is very low.[57] Since SARS-CoV-2's full sequence is more similar to bat than to pangolin coronaviruses,[112] the receptor binding domain of SARS-CoV-2 has been hypothesized to originate from recombination of the relevant portion of pangolin-CoV with an RaTG13-like virus.[113] Temmam et al. wrote that there was no apparent pangolin recombination in the origin of SARS-CoV-2, and that the amino acid sequence similarity in the receptor binding domain was consistent with evolutionary conservation.[17]
Deer mice are highly succeptible to SARS-CoV-2, making them potential reservoir or intermediate hosts.[114] Raccoon dogs could be capable of transmitting SARS-CoV-2 to other animals under farm-like conditions.[106] Transmission between raccoon dogs was shown under laboratory conditions.[103] White-tailed deer are a potential vector for SARS-CoV-2.[106]
Wet market hypothesis[edit]
Spillover has been proposed to have occurred at one or more wet markets in China.[115] Wild and semi-wild game animals are commonly traded and consumed in China, and this practice has expanded in recent decades. The close contact among animals in unsanitary conditions creates the potential for diseases to thrive. In the 2002 outbreak in Guangdong, most living animals in the markets showed serological evidence of exposure to SARS-CoV-1.[108] Pekar et al. hypothesized that similarly to initial outbreaks of SARS-CoV-1 and MERS-CoV, SARS-CoV-2 spilled over to humans from animals at wet markets on multiple separate occasions.[99]

Many early cases were associated with the Huanan seafood market in Wuhan.[21] The first documented COVID-19 infection was in a worker at a seafood stall in the Huanan market.[116] Sequences from that patient have not been published; however, SARS-CoV-2 belonging to lineage B/L were detected in environmental samples from the patient's stall.[116] No bats or pangolins were reported to be at the market between May 2017 and November 2019. Raccoon dogs, which are hypothesized to be succeptible to SARS-CoV-2, were at the market.[117] A survey by He et al. in 2021 identified 102 mammal-affecting viruses in Chinese game animals, 65 of which were identified then for the first time.[118] They did not find any SARS-like viruses, but did find evidence for the transmission of a MERS-like virus from bats to hedgehogs.[118]
Between January 1 and March 30, epidemiologists from China CDC collected 457 samples from animal matter including carcasses and feces and collected 923 environmental samples. All animal samples tested negative for SARS-CoV-2.[119] SARS-CoV-2 was found in 73 environmental samples. Live virus was isolated from three samples, two of which came from stalls belonging to known patients.[119] No significant association was found between environmental virus titer and the type of product sold at particular stalls.[120] SARS-CoV-2 was identified in all four sewer wells in the market. Overground drainage from the surrounding area concentrated under the market.
One environmental sample contained lineage A/S SARS-CoV-2, while all others belonged to lineage B/L.[121] The sample containing lineage A/S also contained evidence of humans and livestock, but no wild animals.[122] Overall, wildlife including racoon dogs were detected at very low levels and mostly associated with negative samples.[123] Liu et al. wrote that there was not enough information to determine the origin of the virus.[123] In particular, they wrote that the evidence does not prove the presence of an infected raccoon dog or the occurrence of multiple zoonotic spillovers at the market as proposed by Pekar et al.
Origins of variants[edit]
The World Health Organization defines variants of concern as a variant with evidence of increase transmissibility, severity, or immune escape.[124] All variants of concern so far independently evolved from the original strain, rather than from each other.[104]
The evolution of the Omicron variant appeared to be more rapid than other variants.[77] Theories for the origin of the omicron variant include long-term circulation among humans outside areas where genetic surveillance was performed, mutation in an immunocompromised individual, or adaptation in an animal species after reverse zoonosis.[125][126] The Omicron variant is proposed to have originated in mice.[61][126]
Views[edit]

Origins of COVID-19 either in the wildlife trade or a laboratory in China would be considered embarrassing to the Chinese government.[127] A number of Chinese publications have argued that COVID-19 originated (in the words of Jon Cohen) "anywhere but here,"[127] by routes including imported frozen food products or competitors in the 2019 Military World Games. Most scientists outside China consider these scenarios to be implausible.[127] Some contributors to the WHO-convened Global Study of Origins of SARS-CoV-2 have said that the resulting report reflected political pressure from the Chinese government.[127] After the WHO-convened report was widely criticized internationally, China withdrew most support for further studies of COVID-19's origins.[127] Scientists including Edward Holmes have expressed skepticism that studies which found no SARS-CoV-2-related viruses in Chinese wildlife were free from political interference.[127] Former China CDC director George Gao expressed willingness to consider any origin theory but stressed a lack of evidence. Gao said, "Once the scientists can do free science, we might get the answer. But now, everything is politicized."[128] News headlines in March 2023 that referred to indications of raccoon dogs at Huanan Seafood Market as the "strongest evidence yet" for a zoonotic origin were criticized by scientists, who said the strength of the conclusions was greatly overstated.[128] Speculation about wet markets and associated culinary practices in the origin of COVID-19 has contributed to racism against Asians.[129][130]
The ODNI reported to the U.S. Congress in June 2023 that "both a natural and laboratory-associated origin remain plausible."[131] The Obama administration had imposed a moratorium on gain-of-function research in 2014 after concerns were raised about safe handling of research materials.[45][132] The moratorium was lifted by the Trump administration in 2017. Scientific experts and officials in the Biden administration have advocated stronger government oversight of pathogen research. Under pressure from the Biden White House as well as congressional Republicans, USAID canceled funding for a program to search for zoonotic pathogens in the wild.[132] Political polarization in the United States has motivated some non-scientific support for both natural and research-related origins of COVID-19.[133]
The competing hypotheses have been fiercely debated on social media. Vociferous advocates of a zoonotic origin have sometimes been called "zoonati", a term that was originally pejorative but that some scientists have chosen to reappropriate.[131][134] They have clashed online with participants in DRASTIC, an internet activist group that has brought to light information about U.S. funding for emerging pathogen research.[134][134] Scientists supporting either origin theory have faced online harassment in response.[134]
A 2020 paper by Andersen et al.[135] was widely cited in news media as conclusively supporting a natural origin.[136] Electronic communications made available in 2021 through Freedom of Information Act requests showed that, in discussion with NIH officials, scientists involved in producing the paper appeared undecided about the virus' origins. Kristian Andersen had written, "the main issue is that accidental release is in fact highly likely—it’s not some fringe theory. I absolutely agree that we can’t prove one way or the other, but we never will be able to—however, that doesn’t mean that by default the data is currently much more suggestive of a natural origin as opposed to e.g. passage. It is not—the furin cleavage site is very hard to explain."[136] Co-author Andrew Rambaut had written, "Given the shitshow that would happen if anyone serious accused the Chinese of even accidental release, my feeling is we should say that given there is no evidence of a specifically engineered virus, we cannot possibly distinguish between natural evolution and escape so we are content with ascribing it to natural processes."[136] In public hearings, some congressional Republicans alleged that the communications evinced undue political influence on the paper's conclusions. Lead author Kristian Andersen described the allegations as "absurd and false", characterizing them as "attacks directed against science and scientists."[136] Andersen said that between the time of the recorded communications and submitting the paper, he changed his mind in light of evidence.[137] Democrats in the hearing defended former NIH director Anthony Fauci and accused Republicans of politicizing science.[136]
Citations[edit]
- Anand, Praveen; Puranik, Arjun; Aravamudan, Murali; Venkatakrishnan, AJ (May 26, 2020). "SARS-CoV-2 strategically mimics proteolytic activation of human ENaC". eLife. 9. doi:10.7554/eLife.58603. PMC 7343387. PMID 32452762.
- Anderson, Kristian G.; Rambaut, Andrew; Lipkin, W. Ian; Holmes, Edward C. (March 17, 2020). "The proximal origin of SARS-CoV-2". Nature. 26 (4): 450–452. doi:10.1038/s41591-020-0820-9. PMC 7095063. PMID 32284615.
- Balloux, François; Tan, Cedric; Swadling, Leo; Richard, Damien (June 20, 2022). "The past, current and future epidemiological dynamic of SARS-CoV-2". Oxford Open Immunology. 3 (1): iqac003. doi:10.1093/oxfimm/iqac003. PMC 9278178. PMID 35872966.
- Berche, Patrick (March 2023). "Gain-of-function and origin of Covid19". La Presse Médicale. 52 (1). doi:10.1016/j.lpm.2023.104167. PMC 10234839. PMID 37269978.
- Biancolella, Michela; Colona, Vito Luigi; Luzzato, Lucio; Watt, Jessica Lee (July 24, 2023). "COVID-19 annual update: a narrative review". Human Genomics. 17 (1): 68. doi:10.1186/s40246-023-00515-2. PMC 10367267. PMID 37488607.
- Cagliani, Rachele; Forni, Diego; Clerici, Mario; Sironi, Manuela (April 1, 2020). "Computational Inference of Selection Underlying the Evolution of the Novel Coronavirus, Severe Acute Respiratory Syndrome Coronavirus 2". Journal of Virology. 94 (12). doi:10.1128/JVI.00411-20. PMC 7307108. PMID 32238584.
- Canuti, Marta; Bianchi, Silvia; Kolbi, Otto; Pond, Sergei L.K. (March 16, 2022). "Waiting for the truth: is reluctance in accepting an early origin hypothesis for SARS-CoV-2 delaying our understanding of viral emergence?". BMJ Global Health. 7 (3): e008386. doi:10.1136/bmjgh-2021-008386. PMC 8927931. PMID 35296465.
- Cohen, Jon (August 19, 2022). "Anywhere but Here". Science. 377 (6608): 805–809. Bibcode:2022Sci...377..805C. doi:10.1126/science.ade4235. PMID 35981032. S2CID 251670601.
- Fernando, Christine; Mumphrey, Cheyanne (December 20, 2020). "Racism targets Asian food, business during COVID-19 pandemic". PBS News Weekend.
- Deng, Shanjun; Xing, Ke; He, Xionglei (February 2022). "Mutation signatures inform the natural host of SARS-CoV-2". National Science Review. 9 (2): nwab220. doi:10.1093/nsr/nwab220. PMC 8690307. PMID 35211321.
- Eban, Katherine (June 1, 2023). "Inside the COVID Origins Raccoon Dog Cage Match". Vanity Fair. Archived from the original on July 1, 2023.
- Frutos, Roger; Pliez, Olivier; Gavotte, Laurent; Devaux, Christian A. (October 6, 2021). "There is no "origin" to SARS-CoV-2". Environmental Research. 207. doi:10.1016/j.envres.2021.112173. PMID 34626592. S2CID 238412878.
- Frutos, Roger; Serra-Cobo, Jordi; Chen, Tianmu; Devaux, Christian A. (August 5, 2020). "COVID-19: Time to exonerate the pangolin from the transmission of SARS-CoV-2 to humans". Infection, Genetics and Evolution. 84. doi:10.1016/j.meegid.2020.104493. PMID 32768565. S2CID 220971074.
- Ge, Xing-Yi; Li, Jia-Lu; Yang, Xing-Lou; Chmura, Aleksei A. (October 30, 2013). "Isolation and characterization of a bat SARS-like coronavirus that uses the ACE2 receptor". Nature. 503 (7477): 535–538. Bibcode:2013Natur.503..535G. doi:10.1038/nature12711. PMID 24172901. S2CID 4464714.
- Goodman, Brenda (July 11, 2023). "Scientists at House hearing refute claims they were bribed and influenced to deny Covid-19 'lab leak' theory". CNN.
- Graham, Rachel L.; Baric, Ralph S. (April 2010). "Recombination, Reservoirs, and the Modular Spike: Mechanisms of Coronavirus Cross-Species Transmission". Journal of Virology. 84 (7): 3134–3146. doi:10.1128/JVI.01394-09. PMC 2838128. PMID 19906932.
- Guo, Hua; Hu, Bing-Jie; Yang, Xing-Lou; Zeng, Lei-Ping (September 29, 2020). "Evolutionary Arms Race between Virus and Host Drives Genetic Diversity in Bat Severe Acute Respiratory Syndrome-Related Coronavirus Spike Genes". Journal of Virology. 94 (20). doi:10.1128/JVI.00902-20. PMID 32699095. S2CID 220716415.
- Guo, Hua; Li, Ang; Dong, Tian-Yi; Su, Jia (November 21, 2022). "ACE2-Independent Bat Sarbecovirus Entry and Replication in Human and Bat Cells". Virology. 13 (6): e0256622. doi:10.1128/mbio.02566-22. PMC 9765407. PMID 36409074.
- Harrison, Neil L.; Sachs, Jeffery D. (May 19, 2022). "A call for an independent inquiry into the origin of the SARS-CoV-2 virus". PNAS. 119 (21): e2202769119. Bibcode:2022PNAS..11902769H. doi:10.1073/pnas.2202769119. PMC 9173817. PMID 35588448.
- He, Wan-Ting; Hou, Xin; Zhao, Jin; Sun, Jiumeng (February 16, 2022). "Virome characterization of game animals in China reveals a spectrum of emerging pathogens". Cell. 185 (7): 1117–1129.e8. doi:10.1016/j.cell.2022.02.014. PMC 9942426. PMID 35298912.
- Holmes, Edward C.; Goldstein, Stephen A.; Rasmussen, Angela L.; Robertson, David L. (September 16, 2021). "The origins of SARS-CoV-2: A critical review". Cell. 184 (19): 4848–4856. doi:10.1016/j.cell.2021.08.017. PMC 8373617. PMID 34480864.
- Hossain, Golzar; Tang, Yan-dong; Akter, Sharmin; Zheng, Chunfu (December 22, 2021). "Roles of the polybasic furin cleavage site of spike protein in SARS-CoV-2 replication, pathogenesis, and host immune responses and vaccination". Journal of Medical Virology. 94 (5): 1815–1820. doi:10.1002/jmv.27539. PMID 34936124. S2CID 245430230.
- Jaimes, Javier A.; Millet, Jean K.; Whittaker, Gary R. (June 26, 2020). "Proteolytic Cleavage of the SARS-CoV-2 Spike Protein and the Role of the Novel S1/S2 Site". iScience. 23 (6). Bibcode:2020iSci...23j1212J. doi:10.1016/j.isci.2020.101212. PMC 7255728. PMID 32512386.
- Kane, Yakhouba; Wong, Gary; Gao, George F. (February 2023). "Animal Models, Zoonotic Reservoirs, and Cross-Species Transmission of Emerging Human-Infecting Coronaviruses". Annual Review of Animal Biosciences. 11: 1–31. doi:10.1146/annurev-animal-020420-025011. PMID 36790890. S2CID 256869353.
- Kang, Lin; He, Guijuan; Sharp, Amanda K.; Wang, Xiaofeng (July 6, 2021). "A selective sweep in the Spike gene has driven SARS-CoV-2 human adaptation". Cell. 184 (17): 4392–4400.e4. doi:10.1016/j.cell.2021.07.007. PMC 8260498. PMID 34289344.
- Kumar, Sudhir; Tao, Qiqing; Weaver, Steven; Sanderford, Maxwell (August 8, 2021). "An Evolutionary Portrait of the Progenitor SARS-CoV-2 and Its Dominant Offshoots in COVID-19 Pandemic". Molecular Biology and Evolution. 38 (8): 3046–3059. doi:10.1093/molbev/msab118. PMID 33942847.
- Li, Hongying; Mendelsohn, Emma; Zong, Chen; Zhang, Wei (September 2019). "Human-animal interactions and bat coronavirus spillover potential among rural residents in Southern China". Biosafety and Health. 1 (2): 84–90. doi:10.1016/j.bsheal.2019.10.004. PMC 7148670. PMID 32501444.
- Li, Meng; Du, Juan; Liu, Weiqiang; Li, Zihao (January 23, 2023). "Comparative susceptibility of SARS-CoV-2, SARS-CoV, and MERS-CoV across mammals". ISME. 17 (4): 549–560. doi:10.1038/s41396-023-01368-2. PMC 9869846. PMID 36690780.
- Liu, William J.; Liu, Peipei; Lei, Wenwen; Jia, Zhiyuan (April 5, 2023). "Surveillance of SARS-CoV-2 at the Huanan Seafood Market". Nature. doi:10.1038/s41586-023-06043-2. PMID 37019149. S2CID 257983307.
- Lytras, Spyros; Hughes, Joseph; Martin, Darren; Swanepoel, Phillip (February 8, 2022). "Exploring the Natural Origins of SARS-CoV-2 in the Light of Recombination". Genome Biology and Evolution. 14 (2). doi:10.1093/gbe/evac018. PMC 8882382. PMID 35137080.
- Lubinski, Bailey; Whittaker, Gary R (August 2023). "The SARS-CoV-2 furin cleavage site: natural selection or smoking gun?". The Lancet Microbe. 4 (8): e570. doi:10.1016/S2666-5247(23)00144-1. PMC 10205058. PMID 37236215.
- MacLean, Oscar A.; Lytras, Spyros; Weaver, Steven; Singer, Joshua B. (March 12, 2021). "Natural selection in the evolution of SARS-CoV-2 in bats created a generalist virus and highly capable human pathogen". PLOS Biology. 19 (3): e3001115. doi:10.1371/journal.pbio.3001115. PMC 7990310. PMID 33711012.
- McCarthy, Simone; Baptista, Eduardo (October 15, 2021). "Science turns nasty in Covid-19 origins argument on Twitter". South China Morning Post. Archived from the original on March 20, 2023.
- Menachery, Vineet D.; Yount, Boyd L., Jr.; Sims, Amy C.; Baric, Ralph S. (March 14, 2016). "SARS-like WIV1-CoV poised for human emergence". PNAS. 113 (11): 3048–3053. Bibcode:2016PNAS..113.3048M. doi:10.1073/pnas.1517719113. PMC 4801244. PMID 26976607.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - Palmer, James (January 27, 2020). "Don't Blame Bat Soup for the Coronavirus". Foreign Policy.
- Pekar, Jonathan E.; Magee, Andrew; Parker, Edyth; Moshiri, Niema (July 26, 2022). "The molecular epidemiology of multiple zoonotic origins of SARS-CoV-2". Science. 377 (6609): 960–966. Bibcode:2022Sci...377..960P. doi:10.1126/science.abp8337. PMC 9348752. PMID 35881005.
- Qian, Zhaohui; Li, Pei; Tang, Xiaolu; Lu, Jian (March 1, 2022). "Evolutionary dynamics of the severe acute respiratory syndrome coronavirus 2 genomes". Medical Review. 2 (1): 3–22. doi:10.1515/mr-2021-0035. PMC 9047652. PMID 35658106.
- Reggiani, Alessandro; Rugna, Gianluca; Bonilauri, Paolo (December 15, 2022). "SARS-CoV-2 and animals, a long story that doesn't have to end now: What we need to learn from the emergence of the Omicron variant". Frontiers in Veterinary Science. 9. doi:10.3389/fvets.2022.1085613. PMC 9798331. PMID 36590812.
- Smith, David (February 28, 2023). ""It's just gotten crazy": how the origins of Covid became a toxic US political debate". The Guardian.
- Tai, Jui-Hung; Sun, Hsaio-Yu; Tsung, Yi-Cheng; Li, Guanghao (August 8, 2022). "Contrasting Patterns in the Early Stage of SARS-CoV-2 Evolution between Humans and Minks". Journal of Virology. 94 (12). doi:10.1128/JVI.00411-20. PMC 7307108. PMID 32238584.
- Temmam, Sarah; Montagutelli, Xavier; Herate, Cécile; Donati, Flora (April 5, 2023). "SARS-CoV-2-related bat virus behavior in the human-relevant models sheds light on the origin of COVID-19". EMBO Reports. 24 (4): e56055. doi:10.15252/embr.202256055. PMC 10074129. PMID 36876574.
{{cite journal}}: CS1 maint: PMC embargo expired (link) - Temmam, Sarah; Vongphayloth, Khamsing; Baquero, Eduard; Munier, Sandie (February 16, 2022). "Bat coronaviruses related to SARS-CoV-2 and infectious for human cells". Nature. 604 (7905): 330–336. Bibcode:2022Natur.604..330T. doi:10.1038/s41586-022-04532-4. PMID 35172323. S2CID 246902858.
- Tobias, Jimmy (July 12, 2023). "Amid Partisan Politicking, Revelations on a Covid Origins Article". The Nation.
- Willman, David (September 7, 2023). "The US quietly terminates a controversial $125m wildlife virus hunting programme amid safety fears". BMJ. 382: 2002. doi:10.1136/bmj.p2002. PMID 37678902. S2CID 261557718.
- Willman, David; Warrick, Joby (April 10, 2023). "Research with exotic viruses risks a deadly outbreak, scientists warn". Washington Post.
- Wu, Yiran; Zhao, Suwen (January 2021). "Furin cleavage sites naturally occur in coronaviruses". Stem Cell Research. 50. doi:10.1016/j.scr.2020.102115. PMC 7836551. PMID 33340798.
- "An acrimonious debate about covid's origins will rumble on". The Economist. June 26, 2023. Archived from the original on November 7, 2023.
Notes[edit]
- ^ :[1] ... "the proximal phylogenetic origin of SARS-CoV-2 and its mode of introduction into human circulation remain unclear. There is no evidence for SARS-CoV-2 having been 'engineered' in a lab. Conversely, the escape of a strain from a lab or an accidental contamination during field work cannot formally be ruled out at this stage. However, a zoonotic spillover event in nature is considered as the most plausible scenario in the scientific community"
- ^ :[2] "The origin of SARS-CoV-2, as well as its mode of introduction into the human population, are unknown at present."
- ^ :[4] "SARS-CoV-2 was speculated to have originated in bats and then jump to the human population via an intermediate animal host. Although viruses similar to SARS-CoV-2, such as BANAL-20-52 derived from the Malayan horseshoe bat (Rhinolophus malayanus), RaTG13 derived from the intermediate horseshoe bat (Rhinolophus affinis), and Pangolin‐CoV present in Malayan pangolins (Manis avania) have been identified, at present the exact animal origin of SARS-CoV-2 remains unclear.":[3] "Because the question of the original host or animal reservoir of SARS-CoV-2 is unresolved, identifying other animals that are highly susceptible to this virus infection is an important task for pandemic control and prevention."
- ^ Harrison and Sachs' analysis of the furin cleavage site have been disputed in a letter to the editor Garry, Robert F. (September 29, 2022). "SARS-CoV-2 furin cleavage site was not engineered". PNAS. 119 (40): e2211107119. Bibcode:2022PNAS..11911107G. doi:10.1073/pnas.2211107119. PMC 9546612. PMID 36173950., with a reply from the authors Harrison, Neil L.; Sachs, Jeffery D. (November 2, 2022a). "Reply to Garry: The origin of SARS-CoV-2 remains unresolved". PNAS. 119 (45): e2215826119. Bibcode:2022PNAS..11915826H. doi:10.1073/pnas.2215826119. PMC 9659384. PMID 36322733.
- ^ :[69] "Weak signs of adaptive evolution during the early phase of the epidemic of SARS-CoV-2 in humans have been observed in many studies (Chaw et al. 2020; Tang et al. 2020; MacLean et al. 2021; Martin et al. 2021)."
- ^ The methodology of Pekar et al. has been questioned in letters from He and Dun; and Frutos et al., available as attachments to the article.
References[edit]
- ^ a b c Balloux et al. 2022, p. 4.
- ^ a b c d e f g h Temmam et al. 2022, p. 330.
- ^ a b c d e Kane, Wong & Gao 2023, p. 15.
- ^ Li et al. 2023, p. 549.
- ^ Cagliani et al. 2020, p. 1.
- ^ a b c d Frutos et al. 2020, p. 1.
- ^ Kane, Wong & Gao 2023, pp. 2–3.
- ^ Kane, Wong & Gao 2023, pp. 12, 23.
- ^ a b Kane, Wong & Gao 2023, p. 12.
- ^ Kane, Wong & Gao 2023, p. 13.
- ^ a b c Kane, Wong & Gao 2023, p. 17.
- ^ Guo et al. 2020.
- ^ Temmam et al. 2022, p. 331,335.
- ^ Temmam et al. 2022, p. 336.
- ^ Lytras et al. 2022.
- ^ a b Qian et al. 2022, p. 6.
- ^ a b c d Temmam et al. 2022, p. 331.
- ^ a b c d Kane, Wong & Gao 2023, p. 3.
- ^ a b Holmes et al. 2021, p. 4850.
- ^ Temmam et al. 2023, p. 8,10.
- ^ a b Lytras et al. 2022, p. 1.
- ^ Lytras et al. 2022, p. 4.
- ^ a b Deng, Xing & He 2022.
- ^ Kane, Wong & Gao 2023, pp. 3, 14.
- ^ a b Cagliani et al. 2020, p. 5.
- ^ a b c d e Wu & Zhao 2021.
- ^ Temmam et al. 2022, p. 334,335.
- ^ a b Hossain et al. 2021, p. 1816.
- ^ Hossain et al. 2021, p. 1817.
- ^ Anand et al. 2020.
- ^ a b c Harrison & Sachs 2022.
- ^ Harrison & Sachs 2022, p. 4.
- ^ Temmam et al. 2022, p. 335.
- ^ Kane, Wong & Gao 2023, p. 23.
- ^ Kane, Wong & Gao 2023, pp. 20, 21, 23.
- ^ Graham & Baric 2010, p. 3135.
- ^ Graham & Baric 2010, pp. 3139–3140.
- ^ Graham & Baric 2010.
- ^ Graham & Baric 2010, pp. 3142.
- ^ Berche 2023, pp. 6–7.
- ^ a b Berche 2023, p. 4.
- ^ Ge et al. 2013.
- ^ Menachery et al. 2016.
- ^ a b Menachery et al. 2016, p. 3052.
- ^ a b Willman & Warrick 2023.
- ^ Li et al. 2019.
- ^ Berche 2023, pp. 4, 8.
- ^ Berche 2023, p. 7.
- ^ Berche 2023.
- ^ Holmes et al. 2021.
- ^ Holmes et al. 2021, p. 4853.
- ^ a b c Tai et al. 2022, p. 9.
- ^ a b Kane, Wong & Gao 2023, p. 14.
- ^ Li et al. 2023.
- ^ a b Li et al. 2023, p. 557.
- ^ a b c d e Biancolella et al. 2023, p. 3.
- ^ a b Frutos et al. 2020, p. 3.
- ^ a b Kane, Wong & Gao 2023, p. 8.
- ^ Kane, Wong & Gao 2023, p. 19.
- ^ Graham & Baric 2010, p. 3139.
- ^ a b Kane, Wong & Gao 2023, p. 20.
- ^ a b Kane, Wong & Gao 2023, p. 4.
- ^ Temmam et al. 2022.
- ^ Qian et al. 2022, p. 8.
- ^ Lytras et al. 2022, p. 3.
- ^ a b c Lytras et al. 2022, p. 6.
- ^ a b c Temmam et al. 2023, p. 10.
- ^ a b Kang et al. 2021, p. 4393.
- ^ Tai et al. 2022, p. 4.
- ^ Tai et al. 2022, p. 8.
- ^ Cagliani et al. 2020, p. 3.
- ^ a b Tai et al. 2022, p. 2.
- ^ Qian et al. 2022.
- ^ Cagliani et al. 2020, p. 7.
- ^ Kane, Wong & Gao 2023, p. 16.
- ^ a b Li et al. 2023, p. 554.
- ^ a b Kane, Wong & Gao 2023, p. 21.
- ^ Kang et al. 2021, p. 4396.
- ^ Kang et al. 2021, p. 4397.
- ^ Qian et al. 2022, p. 11,15.
- ^ Qian et al. 2022, p. 15.
- ^ Lubinski & Whittaker 2023.
- ^ Li et al. 2023, pp. 554–557.
- ^ Tai et al. 2022, p. 5.
- ^ a b Tai et al. 2022, p. 7.
- ^ Qian et al. 2022, p. 10.
- ^ MacLean et al. 2021, pp. 1, 7–9.
- ^ Qian et al. 2022, p. 11.
- ^ Pekar et al. 2022, p. 2.
- ^ a b c Guo et al. 2022.
- ^ Jaimes, Millet & Whittaker 2020, p. 2.
- ^ a b Graham & Baric 2010, p. 3141.
- ^ Temmam et al. 2023, p. 5.
- ^ Temmam et al. 2023, pp. 4–5, 10.
- ^ Canuti et al. 2022, p. 2.
- ^ a b Canuti et al. 2022.
- ^ a b Balloux et al. 2022, p. 2.
- ^ a b Kumar et al. 2021, p. 3051.
- ^ a b Pekar et al. 2022.
- ^ Berche 2023, p. 1,5.
- ^ Berche 2023, p. 5.
- ^ Qian et al. 2022, p. 16.
- ^ a b Reggiani, Rugna & Bonilauri 2022, p. 4.
- ^ a b c d Reggiani, Rugna & Bonilauri 2022, p. 2.
- ^ Tai et al. 2022, p. 2-3.
- ^ a b c Kane, Wong & Gao 2023, p. 11.
- ^ Graham & Baric 2010, pp. 3141, 3142.
- ^ a b Berche 2023, p. 1.
- ^ Frutos et al. 2021.
- ^ Frutos et al. 2020.
- ^ Cagliani et al. 2020, p. 6.
- ^ Temmam et al. 2022, p. 334.
- ^ Kane, Wong & Gao 2023, pp. 19–20.
- ^ Kane, Wong & Gao 2023, p. 10.
- ^ Lytras et al. 2022, p. 2.
- ^ a b Pekar et al. 2022, p. 4.
- ^ Liu et al. 2023, p. 3.
- ^ a b He et al. 2022.
- ^ a b Liu et al. 2023, p. 7.
- ^ Liu et al. 2023, p. 6.
- ^ Liu et al. 2023, pp. 8–9.
- ^ Liu et al. 2023, p. 10.
- ^ a b Liu et al. 2023, p. 12.
- ^ Qian et al. 2022, p. 13.
- ^ Qian et al. 2022, p. 14.
- ^ a b Reggiani, Rugna & Bonilauri 2022, p. 3.
- ^ a b c d e f Cohen 2022.
- ^ a b Eban 2023.
- ^ Fernando & Mumphrey 2020.
- ^ Palmer 2020.
- ^ a b Economist 2023.
- ^ a b Willman 2023.
- ^ Smith 2023.
- ^ a b c d McCarthy & Baptista 2021.
- ^ Anderson et al. 2020.
- ^ a b c d e Tobias 2023.
- ^ Goodman 2023.

