User:Printersmoke/sandbox

Hydrogen bond catalysis is the acceleration of reaction rates by catalysts via hydrogen bond interactions between catalyst and substrate molecules. Hydrogen bond catalysis is a form of noncovalent organocatalysis, and is a relatively recent area of interest in chemistry, compared to methods of activation using Lewis acids or covalent interactions. In biological systems, enzymes often utilize multiple cooperative hydrogen bonds to enable a wide variety of reactions. The network of hydrogen bonds is also crucial in substrate recognition and in fixing the stereochemical outcome of these reactions. Research into new hydrogen bond-catalyzed reactions has progressed with increasing pace since the 1970s. With recent popular interest in stereoselective catalysis, many different types of synthetic catalysts have emerged that effect useful transformations with high rate enhancement, selectivity and under mild conditions. Typically, synthetic catalysts are small organic molecules that feature one or multiple hydrogens bound to electronegative atoms, termed hydrogen bond donors. The mechanism of activation usually involve activation of the electrophile by accepting electron density but may also involve anion-binding, partial protonation, or co-operation with other types of interactions.[1]
Background[edit]
Hydrogen bonding[edit]
The localized attractive interaction between a hydrogen atom (the donor, usually bonded to an electronegative atom), and an electronegative atom from another group (the acceptor) is termed a hydrogen bond. While such attractions are sometimes considered purely electrostatic, they may have quite a bit of covalent character depending on their strength. Crystallographic data and computational studies show that the preferred donor-acceptor angle is 180°, but the degree of directional dependence also depends on the strength of interaction.
Strong hydrogen bonds range from about 14 kcal/mol up to 40 kcal/mol, nearing the strength of covalent interactions. An example is the NH...N interaction in the acid form of proton sponge. In these cases, there is a very strict preference for linearity at hydrogen, and the bonding can be thought of as almost purely covalent. From a donor-acceptor perspective, these bonds can be thought of as donation of a Lewis basic lone pair into the antibonding orbital at the proton. Note that the hydrogen bond donor is the electron acceptor and vice versa. The hydrogen bond length is typically less than 1.5Å, and interactions this strong are rare in catalysis.
Moderate hydrogen bonds range from about 4 kcal/mol to 15 kcal/mol. Theoretical calculations are able to successfully resolve the interaction into terms arising from electrostatic, polarization, charge-transfer, coupling, and exchange repulsion terms and indicate that the attraction is mainly electrostatic in nature. Thus, the linearity condition is significantly relaxed, and XH...Y angles can be bent to as much as 130° while maintaining the hydrogen bond. An example of such interactions is the NH...O=C bond in peptide helices or sheets. The majority of catalysts use hydrogen bonding of moderate strength.
Weak hydrogen bonds are less than 4 kcal/mol in strength, and are entirely electrostatic in character. The assistance of such hydrogen bonds have been invoked in some models and in most cases there is ongoing debate to what extent they are involved.
Enzyme catalysis[edit]

It has been known for several decades that enzymes take advantage of hydrogen bonds to bind substrate molecules, activate them toward a variety of reactions, and impart stereoselectivity. Detailed spectroscopic and kinetic studies of serine proteases have revealed a key step in the catalytic mechanism involving electrophilic activation of the amide carbonyl. Double hydrogen bond donation from an "oxyanion hole" comprising two nearby donors is enough to render an otherwise poor electrophile reactive enough to be hydrolyzed. Computations suggest that the oxyanion hole binds the carbonyl with a strength of 4-7.5 kcal/mol. Double donation is also shown to be important; site-directed mutagenesis of asparagine 155 effects a 300-fold decrease in catalytic rate.
The chorismate mutase-catalyzed Claisen rearrangement of chorismate is an example of a hydrogen bond catalyzed [3,3]-sigmatropic shift. Using a large array of single and double hydrogen bond donors, the substrate is held in a reactive conformation, and the rearrangement occurs both in vivo and in aqueous solution.[2] Electrophilic activation is also thought to be involved since the transition state may have significant allyl cation/enolate anion character. Furthermore, while Claisen rearrangements typically have very negative entropy of activation, the enzyme-catalyzed analogue has a near-zero entropy cost.
Interestingly, nature only rarely uses hydrogen bond catalysis for the purpose of enantioselective reactions. An example of such an activation is the stereoselective aldol reaction catalyzed by Type II aldolase. Site-directed mutagenesis dramatically decreases both the reaction rate and enantioselectivity.
General characteristics[edit]
There are several advantages of hydrogen bond catalysis over Lewis acid catalysis. While organic catalysts almost always exist in their active form, Lewis acid catalysts are often generated in situ because they are water and air sensitive. Furthermore, organocatalysts are generally inexpensive and environmentally friendly, as they are metal-free and can often be synthesized from natural starting materials like amino acids. Most hydrogen bond catalysts also function in milder conditions, and due to their relatively weaker binding, do not suffer from product inhibition. This advantage also distinguishes hydrogen bond catalysis from covalent organocatalysis, wherein the activation involves making strong covalent bonds between substrate and catalyst.
In other respects, hydrogen bond catalysis, at least in its current state, presents marked disadvantages. While Lewis acids can often catalyze reactions extremely low amounts (typically 1 mol% or below), current hydrogen bond catalysts rarely achieve similar rate acceleration and efficiency and can require catalyst loadings of up to 20 mol%. Lewis acids are highly tunable, with simple modification of metal, ligand donicity and sterics, counterion, among other features. Hydrogen bond donors in comparison are not as easily modified, but are still moderately tunable. The hydrogen bond donor ability correlates well with the acidity of the protons. Therefore, we can at least in principle control the degree of hydrogen bonding and steric environment in the catalyst active site by tuning the electronics and structure.
In general, our mechanistic understanding of activation with hydrogen bond catalysts is not as sophisticated as our understanding of enzymatic reactions, since most of these catalyst systems have been developed within the last twenty years. It is however possible to divide the main catalyst designs into several general categories. Most catalysts present either one or two donor protons in a binding site, and these are called single and double hydrogen bond donors respectively. Within these categories there are certain motifs which have emerged as the most popular designs, collectively called privileged fragments or structures. Catalysts which engage in significant partial protonation of the substrate may be referred to as general Brønsted acid catalysts. Recently, many research groups have investigated the combination of hydrogen bond donation with other interactions in bifunctional catalysis, which has generated a large number of new, often enantioselective reactions.
Mechanisms of activation[edit]
Electrophilic activation is one of the major mechanisms by which hydrogen bond donors can activate functional groups such as carbonyls, imines, amides and other polar electrophiles. Coordination of the catalyst can withdraw electron density from the substrate, rendering it more electrophilic. For example, binding of an imine to a urea can lower the LUMO and activate it toward attack by a nucleophile.
Alternatively, hydrogen bonding catalysts can activate substrates through anion-binding.
Single hydrogen bond donors[edit]
Catalysts that participate in a single hydrogen bond interaction with substrates are relatively uncommon compared to the more popular double donor motif. Effective catalysis with single hydrogen bond activation is accompanied by several challenges. Single donors, usually hydroxyl groups, bind substrate molecules less strongly than double donors, and have poor directional control. Thus, it is common to use large, sterically hindered groups on the catalyst if a more rigid catalyst-substrate geometry is required. Another common motif is the use of an intramolecular hydrogen bond to stabilize the intermolecular hydrogen bond.
Biphenols[edit]
In 1985, Jack Hine and coworkers demonstrated one of the first examples of catalysis involving a well-defined catalyst-substrate hydrogen bond using a biphenylenediol.[3] Electrophilic activation of epoxide substrates led to accelerated aminolysis under mild conditions. The same authors showed that a very similar catalyst could catalyze certain Diels-Alder reactions of enones, relying on electrophilic activation of the dienophile.[4]


Although the authors originally proposed a double hydrogen bond, it is now commonly agreed that most diols coordinate through a single hydrogen. The main evidence for this comes from crystallography experiments with TADDOLs and BINOLs, two related classes of catalysts that have since almost completely superseded biphenols as the most popular diol motifs.
TADDOLs[edit]
In 2002, Rawal and colleagues noticed the slight acceleration of hetero-Diels-Alder reactions in protic solvents.[5] Notably, the reaction was ten times faster in CDCl3 than in CDCN, leading the authors to conclude that this was more than a polarity effect. Based on this evidence, a chiral diol catalyst was designed to promote asymmetric hetero-Diels-Alder reactions by hydrogen bond donation and subsequently published in Nature in 2003.[6] The catalyst, based on the α,α,α,α-tetraaryl-1,3-dioxolane-4,5-dimethanol (TADDOL) framework originally popularized as a metal ligand by Dieter Seebach, was able to catalyze the hetero-Diels-Alder reaction of substituted acroleins in high yield and enantiomeric excess. The regular all-carbon asymmetric Diels-Alder reaction was published with the same catalyst system soon afterwards.[7]

A crystal structure of TADDOL shows a strong intramolecular hydrogen bond, rendering it a single hydrogen bond donor. Further evidence of single point activation was obtained from the solid state structure of the TADDOL-acrolein complex, which shows only one hydrogen bond. The electron deficient olefin is thought to engage in a secondary π−π interaction with a proximal naphthyl.[7]

Soon after Rawal's initial work, Yamamoto and colleagues showed the possibility of activating electrophiles other than carbonyls, such as nitroso compounds.[8] Using enamines as nucleophiles, Yamamoto demonstrated the enantioselective activation of arylnitroso electrophiles using Rawal's catalyst, promoting the N-nitroso aldol product selectively. Meanwhile, a different diol catalyst was shown to produce the complementary O-nitroso aldol product.

BINOLs and phosphoric acids[edit]
With the emergence of diols as effective hydrogen bond catalysts, the axial chirality of binaphthalene derivatives began to see application in catalyst designs. The ability of BINOLs to catalyze the Morita-Baylis-Hillman reaction was reported by McDougal and Schaus in 2003[9]. The mechanism of catalysis has not been fully determined. Methylation of one hydroxyl group eliminates enantioselectivity and greatly inhibits catalytic activity, but this is consistent with both single and double hydrogen bond activation. However, it is currently proposed that the mechanism involves single-point binding similar to TADDOL catalysis.

Yamamoto has developed a bis(triflyl)methylbinaphthyl catalyst that is proposed to feature an intramolecular hydrogen bond donated by the bis(triflyl)methyl group. The intramolecular hydrogen bond leaves the hydroxyl proton to serve as a single hydrogen bond donor. Yamamoto's catalyst is a competent catalyst for the Mannich reaction, which is also further evidence of the plausibility of single-point activation with BINOL catalysts[10].
A further class of BINOL derivatives that has found use in catalysis are axially chiral phosphoric acids. Originally, research in this class of compounds had established its utility in molecular recognition. For many years, the primary method of preparing enantioenriched BINOLs had been diastereoselective cocrystallization of the BINOL-derived phosphoric acid with Chinchona alkaloids[11]. The catalysts presumably work by protonation of substrate to form a chiral ion pair. While catalysis involving nearly complete protonation is not traditionally considered hydrogen bond catalysis, enantioselective protonation catalysts feature similar design principles to hydrogen bond catalysts. Examples of reactions that are effectively catalyzed by phosphoric acids include Mannich reactions, aza-Friedel-Crafts reactions of furans, amidoalkylations of diazocarbonyl compounds, asymmetric hydrophosphonylation of aldimines and transfer hydrogenations[12].
Double hydrogen bond donors[edit]
The largest group of hydrogen bond catalysts feature sites that bind through the simultaneous donation of two hydrogen bonds.[13] As in the biological oxy-anion hole strategy, two-point binding has been proven to be a highly successful strategy for electrophilic activation. Compared to single hydrogen bond donors, these interactions are stronger and more directional. Because the attraction is dominantly electrostatic, any Lewis basic site should beable to engage in bifurcated hydrogen bonding with these catalysts. This is a distinct advantage over analogous multi-dentate Lewis acids catalysts, which are often only able to bind to limited types of substrates. A wide variety of functional groups, including aldehydes, ketones, esters, imines, N-acyliminium ions and nitro compounds have been activated with double hydrogen bond catalysis.[12]
Ureas and thioureas[edit]
By far the most popular structural motif in double hydrogen bond activation
Guanidinium ions[edit]
Guanidinium ions, resulting from the protonation of guanidines are structural relatives of ureas and thioureas and are also capable of engaging in double hydrogen bond interactions. The natural amino acid arginine contains a guanidinium functional group that can frequently donate hydrogen bonds in biological catalysis. By virtue of its positive charge, these species are typically stronger donors than neutral thioureas and ureas, and thus have recently been investigated for their potential as catalysts. The positive charge may become a liability on account of its ability to non-productively bind with counteranions or encourage specific rather than general acid pathways.

Corey and Grogan have reported pioneering work in guanidinium catalysis with a bicyclic guanidine capable of catalyzing the asymmetric Strecker reaction of N-benzhydryl imines in high yield[14]. Protonation of the guanidine is thought to generate a guanidinium cyanide that undergoes attack on the imine, which is electrophilically activated by the catalyst. Originally, Corey's paper proposes a single hydrogen bond to the imine substrate, but did not rule out the possibility of two-point binding. In fact, there is support for the latter possibility in the crystal structure of a complex of benzoic acid with the guanidinium catalyst in question. The benzhydryl group is found to be necessary for effective enantioinduction, suggesting the participation of an assisting pi-stacking interaction in the transition state.
Since then, guanidinium catalysts have been shown to catalyze a variety of reactions such as the Henry reaction, Michaael reaction of nitroalkanes, Michael reaction of esters, aza-, phospha- and oxa-Michael additions, asymmetric protonation steps and Mannich reactions[15]. Guanidiniums can also stabilize the transition state of pericyclic reactions such as the Claisen rearrangement[16]. The guanidinium is proposed to stabilize the oxyallyl fragment in the transition state of the rearrangement.
Amidinium ions[edit]
Göbel has researched the rate-acceleration of Diels-Alder reactions using amidinium ions[17]. Initial studies showed the importance of extremely weakly-coordinating anions such as tetrakis(3,5-bis(trifluoromethyl)phenyl)borate. Intermolecular Diels-Alder reactions were successfully catalyzed and asymmetric catalysts could induce moderate enantioselectivity. Later, Johnston developed chiral amidine catalysts for the nitro-Mannich reaction that was capable of inducing high enantioselectivity in reactions with N-boc imines[18] .
Multifunctional catalysts[edit]
Bi- and multifunctional catalysis refer to the combination of two or more simultaneous sites of activation in the transition state. Many bifunctional derivatives of common hydrogen bond donor motifs have emerged over the last decade, including bifunctional thioureas, which have been the most popular class by far. One strategy is to use multiple hydrogen bonding sites to simultaneous activate nucleophile and electrophile. Often, the companion functionality is a Lewis base, a hydrogen bond acceptor, which can act as a specific or general base to activate the nucleophile. This also gives increased control over the relative geometry of the nucleophile and electrophile partners and can induce greater stereoselectivity. An alternative strategy is the use of interactions that stabilize the transition state; the most successful so far is the use of cation-pi interactions to promote cationic cyclization. Finally, Schreiner and others have suggested that several aryl C-H hydrogen bond donors play a significant role in controlling catalyst and substrate geometry in certain catalysts. This is still not fully understood and a discussion of relevant evidence is provided below.
Catalysts which bind to substrate primarily through stronger interactions such as covalent bonds or Lewis acid-base interaction, but which still rely on significant hydrogen bonding are discussed in the next section on minor hydrogen bond interactions.
Multiple-site hydrogen bond donors[edit]
Lewis basic groups[edit]
Cation-pi interactions[edit]
C-H hydrogen bonding[edit]
Other catalysts assisted by hydrogen bonding[edit]
Proline catalysts[edit]
Hydrogen bond interactions are thought to play an important role in mechanism of proline catalysts. The ability of proline to catalyze intramolecular aldol reactions (see Hajos–Parrish–Eder–Sauer–Wiechert reaction) is one of the earliest examples of enamine catalysis[19]. Barbas and List have studied the intermolecular variant of this reaction [20]. There are several proposed mechanisms that account for the stereoselectivity of the Hajos-Parrish reaction. The Agami mechanism (1984) has an enamine intermediate with two proline units involved in the transition state (based on experimental reaction kinetics)[21]. However, Houk's theoretical studies in 2001 show that there is likely a cyclic transition state with the proline carboxyl group engaged in a hydrogen bond, which is key in the stereochemical control[22].

Oligopeptide catalysts[edit]
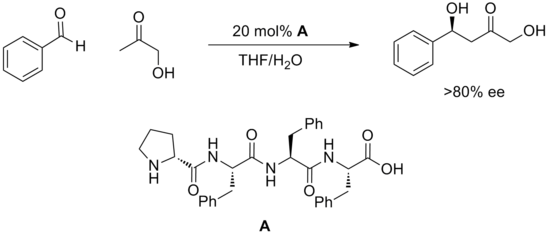
In the past decade, the concept of using synthetic peptides to do catalysis has begun to flourish. While the structural and mechanistic aspects of peptide catalysis are not well understood in general, it is well known that hydrogen bonding plays a major role not only in binding substrate but also in enforcing the geometry of peptide backbone. Reactions that have been successfully catalyzed by synthetic peptides include hydrocyanation, acylation, conjugate additions, aldehyde-imine couplings, aldol reaction and bromination[23].
Oxazaborolidine catalysts[edit]
Titanium Lewis acid catalysts[edit]
Synthetic applications[edit]
To date, there have been few examples of hydrogen bond catalysis in the synthesis of natural products despite the large number of asymmetric reactions discovered. Generally, with high required catalyst loading and catalyst specificity, hydrogen bond catalysis is not yet developed enough to provide a significant improvement over traditional methods. Typically in these examples organocatalysis is used in the beginning stages to quickly access early intermediates with high enantiomeric enrichment.
(+)-Yohimbine (Jacobsen)[edit]
In 2007, Jacobsen reported an enantioselective synthesis of the indole alkaloid yohimbine[24] . The synthesis featured an enantioselective Pictet-Spengler reaction from 1, which was formed from tryptamine. A pyrrole-substituted thiourea catalyst was used in conjunction with acetyl chloride to produce the Pictet-Spengler product in 94% enantiomeric excess. Subsequently, cleavage of the acetyl protecting group with ammonia borane and base was followed by reductive amination with aldehyde A. Protection of the indole nitrogen gives 4, which undergoes Lewis-acid-catalyzed intramolecular Diels-Alder reaction to give the endo product 5. Deprotection and catalytic hydrogenation yields enantiopure (+)-yohimbine.

(-)-Epibatidine (Takemoto)[edit]
In 2008, Takemoto and co-workers disclosed a concise synthesis of the natural alkaloid (-)-epibatidine,[25] relying on a chiral thiourea-catalyzed Michael cascade. Initial asymmetric Michael addition to β-nitrostyrene 1 leads to intermediate 3, which undergoes intramolecular Michael addition to form the cyclic β-ketoester 4 in 77% yield and 75% ee. Palladium-catalyzed decarboxylation, followed by selective reduction of the ketone gives 5 in 71% yield over the two steps. From here, elimination of methanol and 1,4-hydride reduction with sodium cyanoborohydride yields 6, which yields the desired natural product upon mesylation of the alcohol and reduction of the nitro group with zinc dust.

STOP
Hydrogen bond catalysis is the use of hydrogen bonding interactions to accelerate and control reactions. The term hydrogen bond catalysis encompasses a variety of strategies for promoting reactions. Typically, a catalyst contains one or more hydrogen bond donor sites, through which substrate molecules are coordinated. During the reaction hydrogen bond donation can stabilize anionic intermediates resulting from typical nucleophile-electrophile reactions, as well as negatively charged fragments in transition states. Hydrogen bond catalysts can also work through anion-binding mechanisms, in which the catalyst binds to a small anion and forms a closely associated ion pair with the substrate, or general acid mechanisms, in which the catalyst partially transfers a proton to substrate.
Catalytic strategies[edit]
Stabilization of tetrahedral intermediates[edit]
Many useful organic reactions involve the formation of tetrahedral intermediates through nucleophilic attack of functional groups such as aldehydes, amides or imines. In these cases, catalysis with hydrogen bond donors is an attractive strategy since the anionic tetrahedral intermediates are better hydrogen bond acceptors than the starting compound.

For example, in a typical acyl substitution reaction, the starting carbonyl compound is coordinated to the catalyst through one, two or possibly more hydrogen bonds. During the attack of the nucleophile, negative charge builds on the oxygen until the tetrahedral intermediate is reached. Therefore, the formally negative oxygen engages in a much stronger hydrogen bond than the starting carbonyl oxygen because of its increased negative charge. Energetically, this has the effect of lowering the intermediate and the transition state, thus accelerating the reaction.
This mode of catalysis is found in many enzymes, such as the serine proteases. In this example, the amide carbonyl is coordinated to two N-H donors. These sites of multiple coordination designed to promote carbonyl reactions in biology are termed “oxyanion holes”. Delivery of serine nucleophile forms a tetrahedral intermediate, which is stabilized by the increase hydrogen bonding to the oxyanion hole.

Many synthetic catalysts have been able to successfully employ this strategy to activate a variety of electrophiles. Using a chiral BINOL catalyst, for instance, the Morita-Baylis-Hillman reaction involving the addition of enones to aldehydes can be effected with high enantioselectivity. The nucleophile is an enolate-type species generated from the conjugate addition of PEt3 to the enone, and adds enantioselectively to the aldehyde coordinated to catalyst.

In addition to carbonyls, other electrophiles such as imines can be successfully used. For example, using a simple chiral thiourea catalyst, the asymmetric Mannich reaction of aromatic imines with silyl ketene acetals can be catalyzed with high ee in near quantitative conversion. The mechanism of this reaction is not fully resolved and the reaction is very substrate-specific, only effective on certain aromatic electrophiles.

The scope of this mode of activation is immense, with constant new reports of different combinations of electrophiles, nucleophiles and catalyst structures. Furthermore, analogous reactions involving oxyanion intermedates such as enolate addition to nitroso compounds or opening of epoxides have also been successfully catalyzed with this strategy.

However, despite the number of different reactions known, general understanding of the mode of catalysis is limited. Almost all reactions discovered are extremely substrate specific, for reasons that are unclear. Despite detailed mechanistic studies of a few of the systems, overall understanding of the mechanism is much undeveloped. More general challenges are mentioned in the future directions section below.
Stabilization of anionic fragments[edit]
Another strategy that has been explored is the stabilization of reactions that develop partial negative charges in the transition state. Examples of successful applications are most commonly reactions that are approximated concerted and pericyclic in nature. During the course of the reaction, one fragment develops partial negative character and the transition state can be stabilized by accepting hydrogen bond(s).
A demonstrative example is the catalysis of Claisen rearrangements of ester-substituted allyl vinyl ethers reported by the Jacobsen research group. A chiral guanidinium catalyst was found to successfully promote the reaction near room temperature with high enantioselectivity. During the transition state, the fragment coordinated to the amidinium catalyst develops partial anionic character due to the electronegativity of the oxygen and the electron-withdrawing ester group. This increases the strength of hydrogen bonding and lowers the transition state energy, thus accelerating the reaction.

Similarly, negative charge can develop in cycloaddition reactions such as the Diels-Alder reaction, when the partners are appropriately substituted. As a representative example, Rawal and coworkers developed a chiral catalyst based on α,α,α,α-tetraaryl-1,3-dioxolane-4,5-dimethanol (TADDOL) that could catalyze Diels-Alder reactions. In the following example, the reaction with a highly electron-rich diene and an electron-poor dienophile is thought to develop significant negative charge on the enal fragment, and is the transition state is stabilized by increased hydrogen bonding to the TADDOL (Ar = 1-naphthyl).

Anion binding[edit]
Hydrogen bond catalysts can also accelerate reactions by assisting in the formation of electrophilic species through abstracting and/or coordinating an anion such as a halide. Anion-binding catalysis proceeds through an intermediate ion pair. Urea and thiourea catalysts are the most common donors in anion-binding catalysis, and their ability to bind halides and other anions has been well established in the literature. The use of chiral anion-binding catalysts can create an asymmetric ion pair and induce remarkable stereoselectivity.
One of the first reactions proposed to proceed through anion-binding catalysis is the Pictet-Spengler-type cyclization of hydroxylactams with TMSCl under thiourea catalysis. In the proposed mechanism, after initial substitution of the hydroxyl group with chloride, the key ion pair is formed. The activated iminium ion is closely associated with the chiral thiourea-bound chloride, and intramolecular cyclization proceeds with high stereoselectivity.

Asymmetric ion pairs can also be attacked in intermolecular reactions. In an interesting example, asymmetric addition of enol silane nucleophiles to oxocarbenium ions can be effected by catalytically forming the oxocarbenium through anion binding. Starting from an acetal, the chloroether is generated with boron trichloride and reacted with the enol silane and catalyst. The mechanism of formation of the oxocarbenium-thiourea-chloride complex is not fully resolved. It is thought that under the reaction conditions, the chloroether can epimerize and thiourea can stereoselectively bind chloride to form a closely-associated ion pair. This asymmetric ion pair is then attacked by the silane to generate alkylated product.

A notable example of the anion-binding mechanism is the hydrocyanation of imines catalyzed by Jacobsen’s amido-thiourea catalyst depicted in the below diagram. This reaction is also one of the most extensively studied through computational, spectroscopic, labeling and kinetic experiments. While direct addition of cyanide to a catalyst-bound imine was considered, an alternative mechanism involving formation of a iminium-cyanide ion pair controlled by catalyst was calculated to have a barrier that is lower by 20 kcal/mol. The proposed most likely mechanism begins with binding of the catalyst to HNC, which exists in equilibrium with HCN. This complex then protonates a molecule of imine, forming an iminium-cyanide ion pair with the catalyst binding and stabilizing the cyanide anion. The iminium is thought to also interact with the amide carbonyl on the catalyst molecule (see bifunctional catalysis below). The bound cyanide anion then rotates, and attacks the iminium through carbon. The investigators conclude that though imine-urea binding was observed through spectroscopy and was supported by early kinetic experiments, imine binding is off-cycle and all evidence points toward this mechanism involving thiourea-bound cyanide.

Protonation[edit]
It is often difficult to distinguish between hydrogen-bond catalysis and general acid catalysis. Hydrogen bond donors can have varying acidity, from mild to essentially strong Brønsted acids like phosphoric acids. Looking at the extent of proton transfer over the course of the reaction is challenging and has not been investigated thoroughly in most reactions. Nevertheless, strong acid catalysts are often grouped with hydrogen bond catalysts as they represent on extreme on this continuum and share mechanistic similarities. The mechanism of activation for these reactions involves initial protonation of the electrophilic partner. This has the effect of rendering the substrate more electrophilic and creating an ion pair, through which it is possible to transfer stereochemical information.

Asymmetric catalysis involving nearly complete protonation of substrate has been effective in Mannich reactions, aza-Friedel-Crafts reactions of furans, amidoalkylations of diazocarbonyl compounds, asymmetric hydrophosphonylation of aldimines and transfer hydrogenations, among others. Chiral Brønsted acids are often easily prepared from chiral alcohols such as BINOLs, and many are already present in the literature due to their established utility in molecular recognition research.
Multifunctional strategies[edit]
One of the main advantages of hydrogen bond catalysis is the ability to construct catalysts that engage in multiple non-covalent interactions to promote the reaction. In addition to using hydrogen bond donors to activate or stabilize a reactive center during the reaction, it is possible to introduce other functional groups, such as Lewis bases, arenes, or addition hydrogen bonding sites to lend additional stabilization or to influence the other reactive partner.
For instance, the natural enzyme chorismate mutase, which catalyzes the Claisen rearrangement of chorismate, features many other interactions in addition to the hydrogen bonds involved in stabilizing the enolate-like fragment, which is an example of the anionic fragment stabilization strategy discussed above. A key interaction is the stabilization of the other cationic allyl fragment through a cation-pi interaction in the transition state. The use of many additional hydrogen bonds has several putative purposes. The stabilization of multiple hydrogen bonds to the enzyme helps overcome the entropic cost of binding. Additionally, the interactions help hold the substrate in a reactive conformation, and the enzyme-catalyzed reaction has near-zero entropy of activation, while typical Claisen rearrangements in solution have very negative entropies of activation.

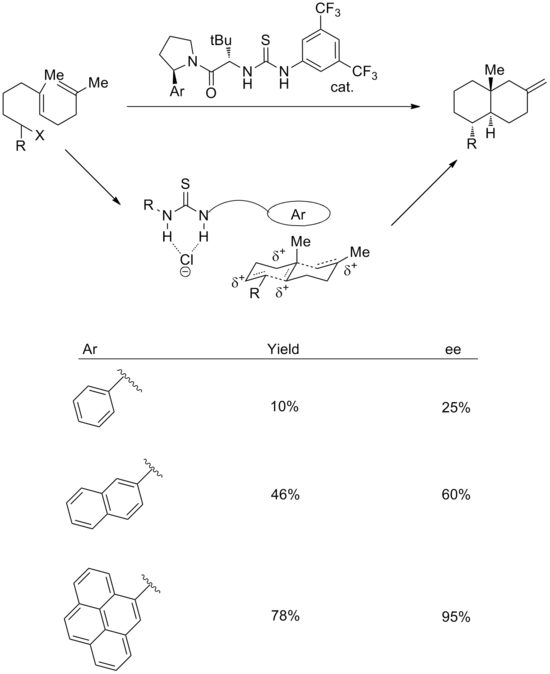
The use of cation-pi interactions has also been successfully implemented in reactions with synthetic catalysts. A combination of anion-binding and cation-pi strategies can be used to effect enantioselective cationic polycyclizations. In the transition state, it is proposed that the thiourea group binds chloride, while the aromatic system stabilizes the associated polyene cation. In support of this, increasing the size of the aromatic ring leads to improvements both in yield and stereoselectivity. The enantioselectivity correlates well with both the polarizability and the quadrupole moment of the aryl group.
Since such a large number of catalysts and reactions involve binding to electrophiles to stabilize the transition state, many bifunctional catalysts also present a Lewis-basic, hydrogen bond acceptor site. As a representative example, Deng and coworkers have developed a thiourea-amine catalyst capable of promoting stereoselective Michael reactions. In the proposed transition state, one of the thiourea N-H donors is coordinated to the Michael acceptor and will stabilize the negative charge buildup. The basic nitrogen lone pair acts as a hydrogen bond acceptor to coordinate the nucleophile, but in the transition state acts as a general base to promote the nucleophilic enolate addition.

This motif of engaging both the nucleophilic and electrophilic partners in a reaction and stabilizing them in the transition state is very common in bifunctional catalysis and many more examples can be found in the article on thiourea organocatalysis.
A relatively new strategy of using synthetic oligopeptides to perform catalysis has yielded many successful examples of catalytic methods. Peptides feature multiple potential sites for hydrogen bonding and it is generally not understood how these engage substrate or how they promote reaction. Peptides have the advantage of being extremely modular and often these catalysts are screened in large arrays. Highly enantioselective reactions have been discovered in this manner such as the aldol reaction depicted below.

Other transformations successfully catalyzed by synthetic peptides include hydrocyanation, acylation, conjugate additions, aldehyde-imine couplings, aldol reaction and bromination. Although the nature of the transition states is unclear, in many examples small changes in the catalyst structure have dramatic effects on reactivity. It is hypothesized that a large number of hydrogen bonds both within the peptide and between catalyst and substrate must cooperate to meet the geometrical requirements for successful catalysis. Beyond this, understanding of catalyst design and mechanism has not yet progressed beyond requiring the testing of libraries of peptides.
Catalyst design[edit]
Privileged structures[edit]
The types of hydrogen bond donors used in catalysis vary widely from reaction to reaction, even among similar catalytic strategies. While specific systems are often studied and optimized extensively, a general understanding of the optimal donor for a reaction or the relationship between catalyst structure and reactivity is greatly lacking. It is not yet possible to rationally design structures to promote a desired reaction with the desired selectivity. However, contemporary hydrogen bond catalysis is primarily focused on a few types of systems that experimentally seem to be effective in a variety of situations. These are termed “privileged structures”.
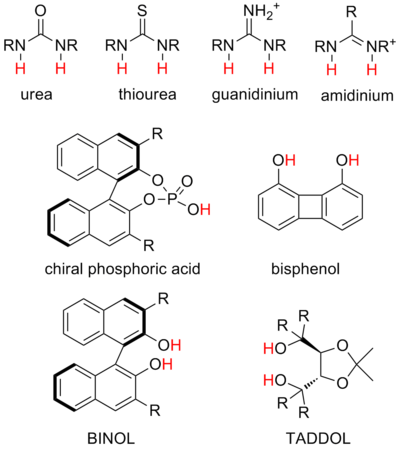
Ureas and thioureas are by far the most common structures and can stabilize a variety of negatively-charged intermediates, as well as engage in anion-binding catalysis. Bifunctional urea and thiourea catalysis are abundant in the literature.
Guanidinium and amidinium ions are structural relatives of ureas and thioureas and can catalyze similar reactions but, by virtue of their positive charge, are stronger donors and much more acidic. The mechanism of guanidinium and amidinium catalysis is thought to often involve partial protonation of substrate.
Diol catalysts are thought to engage substrate with a single hydrogen bond, with the other hydroxyl participating in an internal hydrogen bond. These are some of the earliest hydrogen bond catalysts investigated. They are most commonly used in stabilizing partial anionic charge in transition states, for example coordinating to aldehyde dienophiles in hetero-Diels-Alder reactions.
Phosphoric acid catalysts are the most common strong acid catalysts and work by formation of chiral ion pairs with basic substrates such as imines.
Catalyst tuning[edit]
In general, acidity of donor sites correlates well with the strength of the donor. For example, it is a common strategy to add electron-withdrawing aryl substituents on a thiourea catalyst, which can increase its acidity and thus the strength of its hydrogen bonding. However, it is still unclear how donor strength correlates with desired reactivity. Importantly, more acidic catalysts are not necessarily more effective. For instance, ureas are less acidic than thioureas by roughly 6 pKa units, but it is not generally true that ureas are significantly worse are catalyzing reactions.
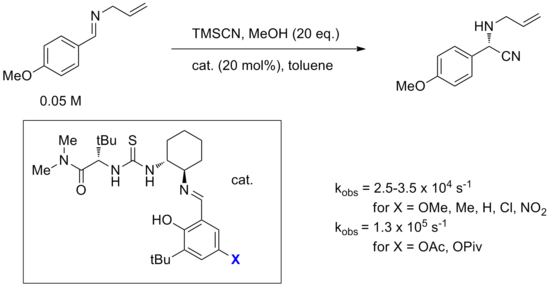
Furthermore, the effect of varying substituents on the catalyst is rarely well-understood. Small substituent changes can completely change reactivity or selectivity. An example of this was in the optimization studies of a bifunctional Strecker reaction catalyst, one of the first well-studied thiourea catalysts. Specifically, varying the X substituent on the salicylaldimine substituent, it was found that typical electron-withdrawing or electron-donating substituents had little effect on the rate, but ester substituents such as acetate or pivaloate seemed to cause noticeable rate acceleration. This observation is difficult to rationalize given that the X group is far from the reactive center during the course of the reaction and electronics do not seem to be the cause. In general, despite the relative ease of electronic tuning with organic catalysts, chemists have not yet reached a useful understanding of these modifications.
Synthetic applications[edit]
Natural product synthesis[edit]
To date, there have been few examples of hydrogen bond catalysis in the synthesis of natural products despite the large number of reactions being discovered. Generally, with high required catalyst loading and often extreme substrate specificity, hydrogen bond catalysis is not yet developed enough to provide useful, general reactions that represent a significant improvement over traditional methods. In the published examples, hydrogen bond catalysis is mainly used in the beginning stages to quickly access early intermediates with high enantiomeric enrichment.
Two illustrative examples are shown below. In the Jacobsen synthesis of (+)-yohimbine, an indole alkaloid, an early enantioselective Pictet-Spengler reaction using a pyrrole-substituted thiourea catalyst produced gram-scale quantities of product in 94% ee and 81% yield. The remainder of the synthesis was short, using a reductive amination and an intramolecular Diels-Alder reaction.

In 2008, Takemoto disclosed a concise synthesis of (-)-epibatidine that relied on a Michael cascade, catalyzed by a bifunctional catalyst. After initial asymmetric Michael addition to the β-nitrostyrene, intramolecular Michael addition furnishes the cyclic ketoester product in 75% ee. Standard functional group manipulations and an intramolecular cyclization yields the natural product.

Scaleable synthesis of building blocks[edit]
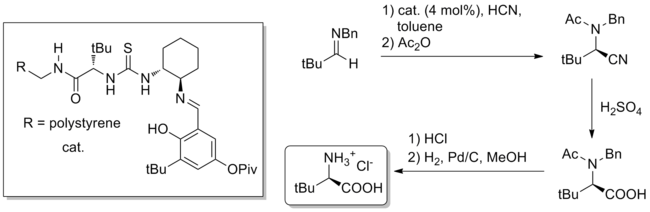
Aside from total synthesis, a potentially useful application of hydrogen bond catalysis is the bulk synthesis of difficult-to-access chiral small molecules. A notable example is the gram-scale Strecker synthesis of unnatural amino acids using thiourea catalysis, reported in the journal Nature in 2009. The catalyst, whether polymer-bound or homogeneous, is derived from natural tert-leucine and can catalyze (4 mol% catalyst loading) the formation of the Strecker product from benzhydryl amines and aqueous HCN. Hydrolysis of the nitrile and deprotections produces pure unnatural tert-leucine in 84% overall yield and 99% ee.
Challenges and future goals[edit]
Despite the widespread interest in organocatalysis and the large number of new catalytic systems that are continuously being discovered, progress in the understanding of mechanism and catalyst design in the field of hydrogen bond catalysis is extremely limited. Compared to a more developed field like palladium-catalyzed coupling reactions, hydrogen bond catalysis presents many challenges that have not yet been successfully tackled>
- Turnover: While palladium-catalyzed reactions can often be effective with catalyst loading of less than 0.1 mol%, hydrogen bond catalysts are often added in more than 10 mol%. Poor rate acceleration is a general trend that will have to be overcome in order for hydrogen bond catalysis to be a practical synthetic strategy.
- Mechanism: In the future, further investigation of the precise steps involved in the mechanism of hydrogen bond catalysis will be required that will enable chemists to rationally design catalytic strategies for more complex or more useful transformations. For comparison, the basic steps of palladium-catalyzed cross coupling have been systematically and thoroughly studied over the last few decades and have led to dramatic advances in catalytic scope, control and reaction design principles. For example, improved understanding of oxidative addition has led to aryl chlorides becoming practical coupling partners, while improved understanding of reductive elimination has led to the development of new reactions involving sp3 centers. Knowing these fundamental catalytic steps, the ability to rationally plan new reactions and cascades has been extremely useful in the field of total synthesis. In contrast, we lack a general, systematic mechanistic understanding of the steps of hydrogen bond catalysis and how to influence them. Detailed mechanistic studies have so far been limited to individual systems, and their findings have not been of demonstrable predictive use.
- Catalyst: A related challenge is the investigation of how changes in catalyst, structural, conformational, and electronic can be used to rationally influence the reaction. The goal would be to fully understand how to use multiple co-operative interactions to best accelerate a reaction and impart selectivity. Ideally, rational catalyst design will eventually replace screening of families of catalysts and the choice of building blocks will become more systematic.
- Scope: While new reactions are constantly being discovered, most reactions have extremely narrow substrate scope and the reason for such narrow scope is often not understood. In the field of palladium catalysis, after the foundations of mechanistic understanding were established, the scope of reactions saw rapid growth. Knowing the factors that affected each step of catalysis allowed for the chemists to evision and pursue new reactions of high synthetic utility, such as C-H bond activation reactions. In the field hydrogen bond catalysis, chemists have not yet reached a stage where new types of reactivity can be easily and systematically targeted. At this point, reaction discovery is useful, but more detailed mechanistic study is required to realize the full potential of hydrogen bond catalysis.
See also[edit]
Further reading[edit]
- Hydrogen Bond Catalysis. Evans Group Meeting Presentation by Peter H. Fuller. Link
- Asymmetric Hydrogen Bond Catalysis. MacMillan Group Meeting Presentation by Anthony Mastracchio. Link
- Hydrogen Bonding in Asymmetric Catalysis. Leighton Group Meeting Presentation by Uttam Tambar. Link
- Asymmetric Catalysis by Chiral Hydrogen-Bond Donors. Wipf Group Meeting Presentation by Zhenglai Fang Link
- Enantioselective Organocatalysis. Ed. Peter I. Dalko, Wiley-VCH: Weinheim, 2007.
- ^ Jacobsen, E. N. (February 2006). "Asymmetric catalysis by chiral hydrogen-bond donors". Angew. Chem. Int. Ed. 45 (10): 1521–1539. doi:10.1002/anie/200503132 (inactive 2023-08-02).
{{cite journal}}: Unknown parameter|coauthors=ignored (|author=suggested) (help)CS1 maint: DOI inactive as of August 2023 (link) CS1 maint: date and year (link) - ^ Knowles, Robert R.; Jacobsen, Eric N. (2010). "Attractive noncovalent interactions in asymmetric catalysis: Links between enzymes and small molecule catalysts". Proc. Nat. Acad. Sci. 107 (48): 20678–20685. doi:10.1073/pnas.1006402107. PMC 2996434. PMID 20956302.
{{cite journal}}: Unknown parameter|month=ignored (help)CS1 maint: date and year (link) - ^ Hine, Jack; Linden, Shwn Meei; Kanagasabapathy, V. M. (1985). "1,8-biphenylenediol is a double hydrogen bonding catalyst for reaction of an epoxide with a nucleophile". J. Am. Chem. Soc. 107 (4): 1082–1083. doi:10.1021/ja00290a067.
{{cite journal}}: Unknown parameter|month=ignored (help)CS1 maint: date and year (link) - ^ Hine, Jack; Linden, Shwn Meei; Kanagasabapathy, V. M. (1985). "Double-hydrogen-bonding catalysis of the reaction of phenyl glycidyl ether with diethylamine by 1,8-biphenylenediol". J. Org. Chem. 50 (25): 5096–5099. doi:10.1021/jo00225a021.
{{cite journal}}: Unknown parameter|month=ignored (help)CS1 maint: date and year (link) - ^ Rawal, Viresh H. (July 2002). "Hydrogen-bond-promoted Hetero-Diels-Alder Reactions of Unactivated Ketones". J. Am. Chem. Soc. 124 (3): 9662–966. doi:10.1021/ja0267627. PMID 12175197.
{{cite journal}}: Unknown parameter|coauthors=ignored (|author=suggested) (help)CS1 maint: date and year (link) - ^ Huang, Y.; Unni, A. K.; Thadani, A. N.; Rawal, V. H. (2003). "Hydrogen bonding: Single enantiomers from a chiral-alcohol catalyst". Nature. 424 (6945): 146. doi:10.1038/424164a (inactive 2023-08-02). PMID 12853945.
{{cite journal}}: Unknown parameter|month=ignored (help)CS1 maint: DOI inactive as of August 2023 (link) CS1 maint: date and year (link) - ^ a b Thadani, Avinash N.; Stankovic, Ana R.; Rawal, Viresh H. (2004). "Enantioselective Diels-Alder reactions catalyzed by hydrogen bonding". PNAS. 101 (16): 5846–5850. doi:10.1073/pnas.0308545101. PMC 395998. PMID 15069185.
{{cite journal}}: CS1 maint: date and year (link) - ^ Momiyama, Norie; Yamamoto, Hisashi (2005). "Bronsted acid catalysis of achiral enamines for regio- and enantioselective nitroso aldol Synthesis". J. Am. Chem. Soc. 127 (4): 1080–1081. doi:10.1021/ja0444637. PMC 1460970. PMID 15669829.
{{cite journal}}: Check date values in:|year=/|date=mismatch (help); Unknown parameter|month=ignored (help) - ^ McDougal, Nolan T.; Schaus, Scott E. (2003). "Asymmetric Morita−Baylis−Hillman reactions catalyzed by chiral Brønsted acids". J. Am. Chem. Soc. 125 (40): 12094–12095. doi:10.1021/ja037705w. PMID 14518986.
{{cite journal}}: Unknown parameter|month=ignored (help)CS1 maint: date and year (link) - ^ Hasegawa, Aiko; Naganawa, Yuki; Fushimi, Makoto; Ishihara, Kazuaki; Yamamoto, Hisashi (2006). "Design of Brønsted acid-assisted chiral Brønsted acid catalyst bearing a bis(triflyl)methyl group for a Mannich-type reaction". Org. Lett. 8 (15): 3175–3178. doi:10.1021/ol060939a. PMID 16836359.
{{cite journal}}: Unknown parameter|month=ignored (help)CS1 maint: date and year (link) - ^ Jansen, A. C. A. (May 1983). "A highly stereoselective synthesis of s(-)-[1,1'-binaphthalene}-2,2'-diol". Tet. Lett. 24 (31): 3261–3262. doi:10.1016/S0040-4039(00)88151-4.
{{cite journal}}: Unknown parameter|coauthors=ignored (|author=suggested) (help)CS1 maint: date and year (link) - ^ a b Doyle, Abigail G.; Jacobsen, Eric N. (2007). "Small-molecule H-bond donors in asymmetric catalysis". Chem. Rev. 107 (12): 5713–5743. doi:10.1021/cr068373r. PMID 18072808.
{{cite journal}}: Unknown parameter|month=ignored (help)CS1 maint: date and year (link) - ^ Schreiner, Peter R. (2003). "Metal-free organocatalysis through explicit hydrogen bonding interactions". Chem. Soc. Rev. 32 (5): 289–296. doi:10.1039/B107298F. PMID 14518182.
- ^ Corey, E. J.; Grogan, Michael J. (1999). "Enantioselective Synthesis of α-Amino Nitriles from N-Benzhydryl Imines and HCN with a Chiral Bicyclic Guanidine as Catalyst". Org. Lett. 1 (1): 157–160. doi:10.1021/ol990623l. PMID 10822552.
{{cite journal}}: Unknown parameter|month=ignored (help)CS1 maint: date and year (link) - ^ Leow, Dasheng (April 2009). "Chiral guanidine catalyzed enantioselective reactions". Chemistry – an Asian Journal. 4 (4): 488–507. doi:10.1002/asia.200800361. PMID 19101939.
{{cite journal}}: Unknown parameter|coauthors=ignored (|author=suggested) (help)CS1 maint: date and year (link) - ^ Uyeda, Christopher; Jacobsen, Eric N. (2011). "Transition-state charge stabilization through multiple non-covalent interactions in the guanidinium-catalyzed enantioselective Claisen rearrangement". J. Am. Chem. Soc. 133 (13): 5062–5075. doi:10.1021/ja110842s. PMC 3070243. PMID 21391614.
{{cite journal}}: Unknown parameter|month=ignored (help)CS1 maint: date and year (link) - ^ Schuster, Tilmann; Bauch, Markus; Dürner, Gerd; Göbel, Michael W. (2000). "Axially chiral amidinium ions as inducers of enantioselectivity in Diels−Alder reactions". Org. Lett. 2 (2): 179–181. doi:10.1021/ol991276i. PMID 10814276.
{{cite journal}}: Unknown parameter|month=ignored (help)CS1 maint: date and year (link) - ^ Nugent, Benjamin M.; Yoder, Ryan A.; Johnston, Jeffrey N. (2004). "Chiral proton catalysis: a catalytic enantioselective direct aza-Henry reaction". J. Am. Chem. Soc. 126 (11): 3418–3419. doi:10.1021/ja031906i. PMID 15025457.
{{cite journal}}: Unknown parameter|month=ignored (help)CS1 maint: date and year (link) - ^ Hajos, Zoltan G.; Parrish, David R. (1974). "Asymmetric synthesis of the bicyclic intermediates of natural product chemistry". J. Org. Chem. 39 (12): 1615–1621. doi:10.1021/jo00925a003.
{{cite journal}}: CS1 maint: date and year (link) - ^ List, B. (2000). "Proline-catalyzed direct asymmetric aldol reactions". J. Am. Chem. Soc. 122: 2395–2396. doi:10.1021/ja994250y (inactive 2023-08-02).
{{cite journal}}: Unknown parameter|coauthors=ignored (|author=suggested) (help)CS1 maint: DOI inactive as of August 2023 (link) - ^ Agami, C. (1984). "Stereochemistry-59 : New insights into the mechanism of the proline-catalyzed asymmetric robinson cyclization; structure of two intermediates. asymmetric dehydration". Tetrahedron. 40 (6): 1031–1038. doi:10.1016/S0040-4020(01)91242-6.
{{cite journal}}: Unknown parameter|coauthors=ignored (|author=suggested) (help) - ^ Bahmanyar, S.; Houk, K. N. (2001). "Transition states of amine-catalyzed aldol reactions involving enamine intermediates: theoretical studies of mechanism, reactivity and stereoselectivity". J. Am. Chem. Soc. 123 (45): 11273–11283. doi:10.1021/ja011403h. PMID 11697970.
{{cite journal}}: CS1 maint: date and year (link) - ^ Wennemers, Helma (2011). "Asymmetric catalysis with peptides". Chem. Commun. 47 (44): 12036–12041. doi:10.1039/C1CC15237H. PMID 21993353.
- ^ Mergott, Dustin J.; Zuend, Stephan J.; Jacobsen, Eric N. (2008). "Catalytic Asymmetric Total Synthesis of (+)-Yohimbine". Org. Lett. 10 (5): 745–8. doi:10.1021/ol702781q. PMID 18257582.
{{cite journal}}: Unknown parameter|month=ignored (help)CS1 maint: date and year (link) - ^ Miyabe, Hideto; Takemoto, Yoshiji (2008). "Discovery and application of asymmetric reaction by multi-functional thioureas". Bull. Chem. Soc. Jap. 81 (7): 785–795. doi:10.1246/bcsj.81.785.
{{cite journal}}: Unknown parameter|month=ignored (help)CS1 maint: date and year (link)
