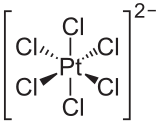User:Praseodymium-141/Platinum compounds
Platinum compounds are compounds that contain the element platinum (Pt). As platinum is a noble metal, it rarely forms compounds.
Halides[edit]

Hexachloroplatinic acid mentioned above is probably the most important platinum compound, as it serves as the precursor for many other platinum compounds. By itself, it has various applications in photography, zinc etchings, indelible ink, plating, mirrors, porcelain coloring, and as a catalyst.[1]
Treatment of hexachloroplatinic acid with an ammonium salt, such as ammonium chloride, gives ammonium hexachloroplatinate,[2] which is relatively insoluble in ammonium solutions. Heating this ammonium salt in the presence of hydrogen reduces it to elemental platinum. Potassium hexachloroplatinate is similarly insoluble, and hexachloroplatinic acid has been used in the determination of potassium ions by gravimetry.[3]
When hexachloroplatinic acid is heated, it decomposes through platinum(IV) chloride and platinum(II) chloride to elemental platinum, although the reactions do not occur stepwise:[4]
- (H3O)2PtCl6·nH2O ⇌ PtCl4 + 2 HCl + (n + 2) H2O
- PtCl4 ⇌ PtCl2 + Cl2
- PtCl2 ⇌ Pt + Cl2
All three reactions are reversible. Platinum(II) and platinum(IV) bromides are known as well. Platinum hexafluoride is a strong oxidizer capable of oxidizing oxygen.
Oxides[edit]
Platinum(IV) oxide, PtO2, also known as "Adams' catalyst", is a black powder that is soluble in potassium hydroxide (KOH) solutions and concentrated acids.[5] PtO2 and the less common PtO both decompose upon heating.[6] Platinum(II,IV) oxide, Pt3O4, is formed in the following reaction:
- 2 Pt2+ + Pt4+ + 4 O2− → Pt3O4
Other compounds[edit]
Unlike palladium acetate, platinum(II) acetate is not commercially available. Where a base is desired, the halides have been used in conjunction with sodium acetate.[7] The use of platinum(II) acetylacetonate has also been reported.[8]
Several barium platinides have been synthesized in which platinum exhibits negative oxidation states ranging from −1 to −2. These include BaPt, Ba
3Pt
2, and Ba
2Pt.[9] Caesium platinide, Cs
2Pt, a dark-red transparent crystalline compound[10] has been shown to contain Pt2−
anions.[11] Platinum also exhibits negative oxidation states at surfaces reduced electrochemically.[12] The negative oxidation states exhibited by platinum are unusual for metallic elements, and they are attributed to the relativistic stabilization of the 6s orbitals.[11]
It is predicted that even the cation PtO2+
4 in which platinum exists in +10 oxidation state may be achievable.[13]
Zeise's salt, containing an ethylene ligand, was one of the first organometallic compounds discovered. Dichloro(cycloocta-1,5-diene)platinum(II) is a commercially available olefin complex, which contains easily displaceable cod ligands ("cod" being an abbreviation of 1,5-cyclooctadiene). The cod complex and the halides are convenient starting points to platinum chemistry.[7]
Cisplatin, or cis-diamminedichloroplatinum(II) is the first of a series of square planar platinum(II)-containing chemotherapy drugs.[14] Others include carboplatin and oxaliplatin. These compounds are capable of crosslinking DNA, and kill cells by similar pathways to alkylating chemotherapeutic agents.[15] (Side effects of cisplatin include nausea and vomiting, hair loss, tinnitus, hearing loss, and nephrotoxicity.)[16][17]
Organoplatinum compounds such as the above antitumour agents, as well as soluble inorganic platinum complexes, are routinely characterised using 195
Pt nuclear magnetic resonance spectroscopy.
-
The hexachloroplatinate ion
-
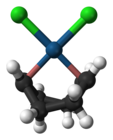
The anion of Zeise's salt
-
Dichloro(cycloocta-1,5-diene)platinum(II)
-
Cisplatin
See also[edit]
References[edit]
- ^ Krebs, Robert E. (1998). "Platinum". The History and Use of our Earth's Chemical Elements. Greenwood Press. pp. 124–127. ISBN 978-0-313-30123-0.
- ^ Kauffman, George B.; Thurner, Joseph J.; Zatko, David A. (1967). Ammonium Hexachloroplatinate(IV). Inorganic Syntheses. Vol. 9. pp. 182–185. doi:10.1002/9780470132401.ch51. ISBN 978-0-470-13240-1.
- ^ Smith, G. F.; Gring, J. L. (1933). "The Separation and Determination of the Alkali Metals Using Perchloric Acid. V. Perchloric Acid and Chloroplatinic Acid in the Determination of Small Amounts of Potassium in the Presence of Large Amounts of Sodium". Journal of the American Chemical Society. 55 (10): 3957–3961. doi:10.1021/ja01337a007.
- ^ Schweizer, A. E.; Kerr, G. T. (1978). "Thermal Decomposition of Hexachloroplatinic Acid". Inorganic Chemistry. 17 (8): 2326–2327. doi:10.1021/ic50186a067.
- ^ Perry, D. L. (1995). Handbook of Inorganic Compounds. Vol. 177. pp. 296–298. Bibcode:1956Natur.177..639.. doi:10.1038/177639a0. ISBN 978-0-8493-8671-8. S2CID 4184615.
{{cite book}}:|journal=ignored (help) - ^ Cite error: The named reference
lagowskiwas invoked but never defined (see the help page). - ^ a b Cite error: The named reference
hanwas invoked but never defined (see the help page). - ^ Ahrens, Sebastian; Strassner, Thomas (2006). "Detour-free synthesis of platinum-bis-NHC chloride complexes, their structure and catalytic activity in the CH activation of methane". Inorganica Chimica Acta. 359 (15): 4789–4796. doi:10.1016/j.ica.2006.05.042.
- ^ Karpov, Andrey; Konuma, Mitsuharu; Jansen, Martin (2006). "An experimental proof for negative oxidation states of platinum: ESCA-measurements on barium platinides". Chemical Communications. 44 (8): 838–840. doi:10.1039/b514631c. PMID 16479284.
- ^ Karpov, Andrey; Nuss, Jürgen; Wedig, Ulrich; Jansen, Martin (2003). "Cs2Pt: A Platinide(-II) Exhibiting Complete Charge Separation". Angewandte Chemie International Edition. 42 (39): 4818–21. doi:10.1002/anie.200352314. PMID 14562358.
- ^ a b Jansen, Martin (2005). "Effects of relativistic motion of electrons on the chemistry of gold and platinum". Solid State Sciences. 7 (12): 1464–74. Bibcode:2005SSSci...7.1464J. doi:10.1016/j.solidstatesciences.2005.06.015.
- ^ Ghilane, J.; Lagrost, C.; Guilloux-Viry, M.; Simonet, J.; et al. (2007). "Spectroscopic Evidence of Platinum Negative Oxidation States at Electrochemically Reduced Surfaces". Journal of Physical Chemistry C. 111 (15): 5701–7. doi:10.1021/jp068879d.
- ^ Gunther, M. (13 June 2016). "Oxidation state +10 may exist in a platinum compound". Chemistry World.
Yu, H.S.; Truhlar, D.G. (2016). "Oxidation State 10 Exists". Angew. Chem. Int. Ed. 55 (31): 9004–6. doi:10.1002/anie.201604670. PMID 27273799. - ^ Riddell, Imogen A.; Lippard, Stephen J. (2018). "Cisplatin and Oxaliplatin:Our Current Understanding of Their Actions". In Sigel, Astrid; Sigel, Helmut; Freisinger, Eva; Sigel, Roland K. O. (eds.). Metallo-Drugs:Development and Action of Anticancer Agents. Vol. 18. pp. 1–42. doi:10.1515/9783110470734-007. ISBN 978-3-11-046984-4. PMID 29394020.
{{cite book}}:|journal=ignored (help) - ^ Richards, A. D.; Rodger, A. (2007). "Synthetic metallomolecules as agents for the control of DNA structure" (PDF). Chemical Society Reviews. 36 (3): 471–483. doi:10.1039/b609495c. PMID 17325786.
- ^ Carinder, James A.; Morrison, Pilar M.; Morrison, David G.; Jack E. Saux III (7 July 2014). Practical Oncology Protocols. Mill City Press, Incorporated. p. 22. ISBN 978-1-62652-816-1. Archived from the original on 9 November 2017. Retrieved 11 June 2016.
- ^ Taguchi, Takashi; Nazneen, Arifa; Abid, M. Ruhul; Razzaque, Mohammed S. (2005). Cisplatin-Associated Nephrotoxicity and Pathological Events. Contributions to Nephrology. Vol. 148. pp. 107–121. doi:10.1159/000086055. ISBN 978-3-8055-7858-5. PMID 15912030. S2CID 24509477.