User:Peak/Test
aUser:Peak/Test/]]b
aUser:Peak/Test/]]b
http://commons.wikimedia.org/wiki/Image:Arr_r.png
commons:commons:Image:Arr_r.png
<img src=http://upload.wikimedia.org/wikipedia/en/2/2d/Arrow_Blue_Right_001.jpeg />
| Peak/Test | |
|---|---|
| Systematic name | (S)-2-Amino-3-phenyl- propanoic acid |
| Abbreviations | Phe F |
| Chemical formula | C9H11NO2 |
| Molecular mass | 165.19 g mol-1 |
| Melting point | 283 °C |
| Density | 1.29 g cm-3 |
| Isoelectric point | 5.5 |
| pKa | 2.20 9.09 |
| CAS number | 63-91-2 |
| SMILES | C1=CC=C(C=C1)CC(C(=O)O)N |

| |
| Disclaimer and references | |
The alpha-amino acid Phenylalanine exists in two forms, the D- and L- forms, which are enantiomers (mirror-image molecules) of each other. It has a benzyl side chain. Its name comes from its chemical structure consisting of a phenyl group substituted for one of the hydrogens in the side chain of alanine. Because of its phenyl group, phenylalanine is an aromatic compound. At room temperature, it is a white, powdery solid.
L-Phenylalanine (LPA) is an electrically neutral amino acid, one of the twenty common amino acids used to biochemically form proteins, coded for by DNA. Its enantiomer, D-phenylalanine (DPA), can be synthesized artificially.
L-phenylalanine is used in living organisms, including the human body, where it is an essential amino acid. L-phenylalanine can also be converted into L-tyrosine, another one of the twenty protein-forming amino acids. L-tyrosine is converted into L-DOPA, norepinephrine, and epinephrine. D-phenylalanine can be converted only into phenylethylamine.
The genetic disorder phenylketonuria is an inability to metabolize phenylalanine.
The synthesized mix DL-Phenylalanine (DLPA), which is a combination of the D- and L- forms, is used as a nutritional supplement.
Phenylalanine is part of the composition of aspartame, a common sweetener found in prepared foods (particularly soft drinks, and gum). Due to phenylketonuria, products containing aspartame usually have a warning label stating that they contain phenylalanine, in compliance with U.S. FDA guidelines.
The genetic codon for phenylalanine was the first to be discovered. Marshall W. Nirenberg discovered that, when he inserted m-RNA made up of multiple uracil repeats into E. coli, the bacterium produced a new protein, made up solely of repeated phenylalanine amino acids.
Phenylalanine uses the same active transport channel as tryptophan to cross the blood-brain barrier, and in large quantities interferes with the production of serotonin.
| Forms of Phenylalanine | |||||
|---|---|---|---|---|---|
| Common name | phenylalanine | D-phenylalanine | L-phenylalanine | DL-phenylalanine | |
| Synonyms | alpha-Amino-beta- phenylpropionic acid |
(2R)-2-amino-3- phenylpropanoic acid |
(2S)-2-amino-3- phenylpropanoic acid |
||
| PubChem | CID 994 from PubChem | CID 71567 from PubChem | CID 6140 from PubChem | ||
| EINECS number | |||||
| CAS number | 673-06-3 | 63-91-2 | 150-30-1 | ||
Biosynthesis[edit]
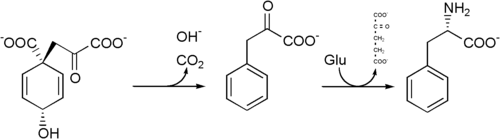
Phenylalanine cannot be made by animals, which have to obtain it from their diet. It is produced by plants and most microorganisms from prephenate, an intermediate on the shikimate pathway.
Prephenate is decarboxylated with loss of the hydroxyl group to give phenylpyruvate. This is transaminated using glutamate as the nitrogen source to give phenylalanine and α-ketoglutarate.
Literal Text[edit]
- 10
- 42 : What happened to 10:42
- 10
- 42 : What happened to {{lc:10:42}}
- 10:42
- What happened to 10{{COLON}}42
- 10
- 42: What happened to 10{{Unicode|:}}42
- 10:42
- What happened to<nowiki>10:42</nowiki>
- 10&58;42
- What happened to 10&58;42
- 10:42
- What happened to 10:42
- 10:42
- What happened to {{nowiki|10:42}}
- 10:42
- What happened to {{black|10:42}}