User:Mr. Ibrahem/Rosiglitazone
 | |
 | |
| Clinical data | |
|---|---|
| Trade names | Avandia, others |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a699023 |
| License data |
|
| Pregnancy category |
|
| Routes of administration | By mouth |
| Drug class | Thiazolidinedione[1] |
| Legal status | |
| Legal status | |
| Pharmacokinetic data | |
| Bioavailability | 99% |
| Protein binding | 99.8% |
| Metabolism | Liver (CYP2C8-mediated) |
| Elimination half-life | 3–4 hours |
| Excretion | Kidney (64%) and fecal (23%) |
| Identifiers | |
| |
| Chemical and physical data | |
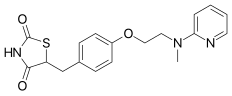
| Formula | C18H19N3O3S |
| Molar mass | 357.43 g·mol−1 |
| 3D model (JSmol) | |
| Chirality | Racemic mixture |
| Melting point | 122 to 123 °C (252 to 253 °F) |
| |
| |
| | |
Rosiglitazone, sold under the brand name Avandia and others, is a medication used to treat type 2 diabetes.[1] Metformin is generally the first line medication; with rosiglitazone worsening outcomes.[1][2] It is taken by mouth.[1] It is also available in combination with metformin or glimepiride.[3]
Common side effects include headache and upper respiratory tract infection.[1] Other side effects may include heart failure, swelling, bone fractures, and liver problems.[1] While there was previous concerns regarding heart attacks, these concerns have not be substantiated.[4][3] Safety in pregnancy is unclear.[5] It is in the thiazolidinedione class and works by increasing sensitivity to insulin.[1]
Rosiglitazone was patented in 1987 and approved for medical use in the United States in 1999.[6][1] While it was approved for use in Europe in 2000, it approval was suspended in 2010 due to safety concerns, and withdrawn in 2015.[7][8][9] It was withdrawn from the market in India in 2010,[10] and New Zealand in 2011.[11] In the United States a month of treatment costs about 150 USD as of 2021.[12]
References[edit]
- ^ a b c d e f g h i j k "Rosiglitazone Monograph for Professionals". Drugs.com. Archived from the original on 25 January 2021. Retrieved 18 October 2021.
- ^ Ton, Joey (4 October 2010). "#34 Rosiglitazone – Reasonable Option or Regrettable Choice?". CFPCLearn. Archived from the original on 2 July 2023. Retrieved 18 June 2023.
- ^ a b Research, Center for Drug Evaluation and (29 June 2021). "FDA Drug Safety Communication: FDA eliminates the Risk Evaluation and Mitigation Strategy (REMS) for rosiglitazone-containing diabetes medicines". FDA. Archived from the original on 30 June 2021. Retrieved 19 October 2021.
- ^ Lebovitz, HE (27 November 2019). "Thiazolidinediones: the Forgotten Diabetes Medications". Current diabetes reports. 19 (12): 151. doi:10.1007/s11892-019-1270-y. PMID 31776781.
- ^ "Rosiglitazone (Avandia) Use During Pregnancy". Drugs.com. Archived from the original on 18 January 2021. Retrieved 19 October 2021.
- ^ Fischer, Jnos; Ganellin, C. Robin (2006). Analogue-based Drug Discovery. John Wiley & Sons. p. 450. ISBN 9783527607495. Archived from the original on 2020-12-21. Retrieved 2021-07-21.
- ^ "Avandia". Archived from the original on 9 April 2021. Retrieved 19 October 2021.
- ^ "EMEA/H/C/000268" (PDF). Archived (PDF) from the original on 19 October 2021. Retrieved 19 October 2021.
- ^ "European Medicines Agency recommends suspension of Avandia, Avandamet and Avaglim". News and Events. European Medicines Agency. 2018-09-17. Archived from the original on 2015-09-24. Retrieved 2022-03-14.
- ^ "Drugs banned in India". Central Drugs Standard Control Organization, Dte.GHS, Ministry of Health and Family Welfare, Government of India. Archived from the original on 2015-02-21. Retrieved 2013-09-17.
- ^ "Diabetes drug withdrawn". Stuff.co.nz. NZPA. 17 February 2011. Archived from the original on 13 October 2013. Retrieved 5 November 2011.
- ^ "Avandia Prices, Coupons & Patient Assistance Programs". Drugs.com. Archived from the original on 23 April 2021. Retrieved 19 October 2021.
