User:Mr. Ibrahem/Olodaterol
 | |
| Clinical data | |
|---|---|
| Pronunciation | Striverdi Respimat /ˈstrɪvərdi ˈrɛspɪmæt/ STRIV-ər-dee RES-pim-at |
| Trade names | Striverdi Respimat |
| AHFS/Drugs.com | Monograph |
| Pregnancy category |
|
| Routes of administration | Inhalation (MDI) |
| Drug class | LABA[1] |
| Legal status | |
| Legal status | |
| Pharmacokinetic data | |
| Bioavailability | ~30% (inhalation)[2] |
| Protein binding | ~60% |
| Metabolism | Liver |
| Elimination half-life | 7.5 hours |
| Excretion | Feces (53%), urine (38%) — following IV administration |
| Identifiers | |
| |
| Chemical and physical data | |
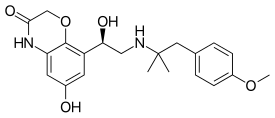
| Formula | C21H26N2O5 |
| Molar mass | 386.448 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
Olodaterol, sold under the brand name Striverdi, is a long-acting β adrenoreceptor agonist (LABA) used to treat chronic obstructive pulmonary disease (COPD).[1] Use is not recommended in asthma.[1] It is breathed into the lungs.[1]
Common side effects include cough, diarrhea, joint pain, rash, and runny nose.[1] Other side effects may include QT prolongation and bronchospasm.[1] In asthma there are concerns regarding an increased risk of death.[1] While there is no evidence of harm in pregnancy, such use has not been well studied.[3]
Olodaterol was approved for medical use in the United States in 2014.[1] It is also available in combination with tiotropium as a single inhaler.[1] In the United Kingdom a month of medication costs the NHS about £26 as of 2021.[4] In the United States this amount costs about 230 USD.[5]
References[edit]
- ^ a b c d e f g h i j k l "Olodaterol Monograph for Professionals". Drugs.com. Archived from the original on 23 January 2021. Retrieved 7 November 2021.
- ^ "Striverdi Respimat (olodaterol) Inhalation Spray For Oral Inhalation. U.S. Full Prescribing Information" (PDF). Boehringer Ingelheim Pharmaceuticals, Inc., Ridgefield, CT 06877 USA. Archived (PDF) from the original on 5 March 2016. Retrieved 23 February 2016.
- ^ "Olodaterol (Striverdi Respimat) Use During Pregnancy". Drugs.com. Archived from the original on 5 December 2020. Retrieved 7 November 2021.
- ^ BNF 81: March-September 2021. BMJ Group and the Pharmaceutical Press. 2021. p. 268. ISBN 978-0857114105.
- ^ "Striverdi Prices, Coupons & Savings Tips - GoodRx". GoodRx. Archived from the original on 15 July 2016. Retrieved 7 November 2021.
