User:Mr. Ibrahem/Nilotinib
 | |
 | |
| Clinical data | |
|---|---|
| Trade names | Tasigna, others |
| Other names | AMN107 |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a608002 |
| License data | |
| Pregnancy category |
|
| Routes of administration | By mouth |
| Drug class | Bcr-Abl tyrosine kinase inhibitor[1] |
| Legal status | |
| Legal status | |
| Pharmacokinetic data | |
| Bioavailability | 30%[2] |
| Protein binding | 98%[2] |
| Metabolism | Liver (mostly CYP3A4-mediated)[2] |
| Elimination half-life | 15-17 hours[2] |
| Excretion | Faeces (93%)[2] |
| Identifiers | |
| |
| Chemical and physical data | |
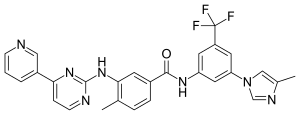
| Formula | C28H22F3N7O |
| Molar mass | 529.527 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| | |
Nilotinib, sold under the brand name Tasigna, is a medication used to treat chronic myelogenous leukemia (CML) which has the Philadelphia chromosome.[3] It may be used both in initial cases of chronic phase CML as well as in accelerated and chronic phase CML that has not responded to imatinib.[3][1] It is taken by mouth.[1]
Common side effects may include low platelets, low white blood cells, anemia, rashes, vomiting, diarrhea, and joint pains.[1] Other serious side effects may include QT prolongation, sudden death, pancreatitis, and liver problems.[1] It is not safe for use during pregnancy.[1] Nilotinib is a Bcr-Abl tyrosine kinase inhibitor and works by interfering with signalling within the cancer cell.[1]
Nilotinib was approved for medical use in the United States in 2007.[1] It is on the World Health Organization's List of Essential Medicines.[5] In the United Kingdom it costs the NHS £2,433 per month as of 2018.[6] In the United States this amount costs US$14,368 as of 2019.[7]
References[edit]
- ^ a b c d e f g h i "Nilotinib Monograph for Professionals". Drugs.com. Archived from the original on 14 July 2021. Retrieved 14 November 2019.
- ^ a b c d e "Tasigna (nilotinib) dosing, indications, interactions, adverse effects, and more". Medscape Reference. WebMD. Archived from the original on 17 July 2021. Retrieved 25 January 2014.
- ^ a b c "Nilotinib". National Cancer Institute. 1 February 2008. Archived from the original on 14 July 2021. Retrieved 14 November 2019.
- ^ "WHOCC - ATC/DDD Index". www.whocc.no. Archived from the original on 25 November 2020. Retrieved 10 September 2020.
- ^ "World Health Organization model list of essential medicines: 21st list 2019" (Document). 2019. hdl:10665/325771.
{{cite document}}: Cite document requires|publisher=(help) - ^ British national formulary : BNF 76 (76 ed.). Pharmaceutical Press. 2018. p. 960. ISBN 9780857113382.
- ^ "Tasigna Prices, Coupons & Patient Assistance Programs". Drugs.com. Archived from the original on 24 December 2019. Retrieved 14 November 2019.
