User:Mr. Ibrahem/Levetiracetam
| |||
| Clinical data | |||
|---|---|---|---|
| Pronunciation | /lɛvɪtɪˈræsɪtæm/ | ||
| Trade names | Keppra, Elepsia, other | ||
| AHFS/Drugs.com | Monograph | ||
| MedlinePlus | a699059 | ||
| License data |
| ||
| Pregnancy category |
| ||
| Routes of administration | By mouth, intravenous | ||
| Drug class | Anticonvulsant (racetam) | ||
| Legal status | |||
| Legal status | |||
| Pharmacokinetic data | |||
| Bioavailability | ~100% | ||
| Protein binding | <10% | ||
| Metabolism | Enzymatic hydrolysis of acetamide group | ||
| Elimination half-life | 6–8 hrs | ||
| Excretion | Urinary | ||
| Identifiers | |||
| |||
| Chemical and physical data | |||
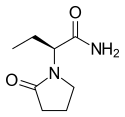
| Formula | C8H14N2O2 | ||
| Molar mass | 170.212 g·mol−1 | ||
| 3D model (JSmol) | |||
| |||
| |||
| | |||
Levetiracetam, marketed under the trade name Keppra among others, is a medication used to treat epilepsy.[1] It is used for partial onset, myoclonic, or tonic-clonic seizures.[1] It is taken by mouth as an immediate or extended release formulation or by injection into a vein.[1]
Common side effects include sleepiness, dizziness, feeling tired, and aggression.[1] Severe side effects may include psychosis, suicide, and allergic reactions such as Stevens–Johnson syndrome and anaphylaxis.[1] It is unclear if use is safe during pregnancy but appears to be safe for use when breastfeeding.[3] It is the S-enantiomer of etiracetam.[4] How it works is not clear.[1]
Approved for medical use in the United States in 1999,[1] levetiracetam is available as a generic medication.[5] A month's supply in the United Kingdom costs the NHS about £19.31 as of 2019.[5] In the United States, the wholesale cost of this amount is about US$4.50.[6] In 2017, it was the 110th most commonly prescribed medication in the United States, with more than six million prescriptions.[7][7]
References[edit]
- ^ a b c d e f g h i "Levetiracetam Monograph for Professionals". Drugs.com. AHFS. Archived from the original on 24 March 2019. Retrieved 14 January 2019.
- ^ "WHOCC - ATC/DDD Index". www.whocc.no. Archived from the original on 23 October 2020. Retrieved 6 September 2020.
- ^ "Levetiracetam Use During Pregnancy". Drugs.com. Archived from the original on 6 March 2019. Retrieved 5 March 2019.
- ^ Cavanna, Andrea E. (2018). Behavioural Neurology of Anti-Epileptic Drugs: A Practical Guide. Oxford University Press. p. 17. ISBN 9780198791577. Archived from the original on 6 March 2019. Retrieved 5 March 2019.
- ^ a b British national formulary: BNF 76 (76 ed.). Pharmaceutical Press. 2018. p. 319. ISBN 9780857113382.
- ^ "NADAC as of 2019-02-27". Centers for Medicare and Medicaid Services. Archived from the original on 6 March 2019. Retrieved 3 March 2019.
- ^ a b "The Top 300 of 2020". ClinCalc. Archived from the original on 12 February 2021. Retrieved 11 April 2020. Cite error: The named reference "ref" was defined multiple times with different content (see the help page).