User:Mr. Ibrahem/Ethyl eicosapentaenoic acid
 | |
| Clinical data | |
|---|---|
| Trade names | Vascepa, Vazkepa, others |
| Other names | Eicosapentaenoic acid ethyl ester; Ethyl eicosapentaenoate; Eicosapent; EPA ethyl ester; E-EPA, Icosapent ethyl (USAN US) |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a613024 |
| License data | |
| Routes of administration | By mouth |
| Drug class | Omega-3 fatty acid[1] |
| Legal status | |
| Legal status | |
| Identifiers | |
| |
| Chemical and physical data | |
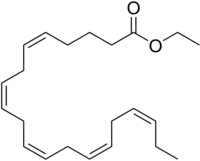
| Formula | C22H34O2 |
| Molar mass | 330.512 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| | |
Ethyl eicosapentaenoic acid (E-EPA), also known as icosapent ethyl, is a medication used to treat high triglycerides.[3][2] It is used with diet and statins when triglycerides are greater than 500 mg/dL; or greater than 150 mg/dL in those at high risk.[2] It is taken by mouth.[4]
Common side effects include muscle pain, swelling, constipation, gout, and atrial fibrillation.[2] Other side effects may include allergic reactions and bleeding.[2] It is made from the omega-3 fatty acid eicosapentaenoic acid (EPA) from fish oil.[1] How it works is not exactly clear.[3]
Ethyl eicosapentaenoic acid was approved for medical use in the United States in 2012 and Europe in 2021.[4][3] A generic version has been approved.[5] In the United States it costs about 84 USD per month as of 2021.[6] As of 2021 it was not commercially available in the United Kingdom or Europe.[7]
References[edit]
- ^ a b "FDA approves use of drug to reduce risk of cardiovascular events in certain adult patient groups". U.S. Food and Drug Administration (FDA) (Press release). 13 December 2019. Archived from the original on 22 December 2019. Retrieved 21 December 2019.
 This article incorporates text from this source, which is in the public domain.
This article incorporates text from this source, which is in the public domain.
- ^ a b c d e f g "Vascepa- icosapent ethyl capsule". DailyMed. 23 December 2019. Archived from the original on 5 February 2021. Retrieved 15 January 2020.
- ^ a b c d e "Vazkepa EPAR". European Medicines Agency (EMA). 25 January 2021. Archived from the original on 9 July 2021. Retrieved 7 July 2021.
- ^ a b "Icosapent Ethyl Monograph for Professionals". Drugs.com. Archived from the original on 9 March 2021. Retrieved 25 November 2021.
- ^ "Icosapent ethyl: FDA-Approved Drugs". U.S. Food and Drug Administration (FDA). Archived from the original on 19 October 2020. Retrieved 15 August 2020.
- ^ "Icosapent Ethyl Prices, Coupons & Savings Tips - GoodRx". GoodRx. Retrieved 25 November 2021.
- ^ "Icosapent ethyl". SPS - Specialist Pharmacy Service. 21 January 2016. Archived from the original on 11 December 2021. Retrieved 25 November 2021.
