User:Mr. Ibrahem/Erdafitinib
 | |
| Clinical data | |
|---|---|
| Trade names | Balversa |
| Other names | JNJ-42756493 |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a619031 |
| License data |
|
| Routes of administration | By mouth |
| Drug class | Fibroblast growth factor receptor (FGFR) blocker[1] |
| Legal status | |
| Legal status |
|
| Identifiers | |
| |
| Chemical and physical data | |
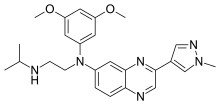
| Formula | C25H30N6O2 |
| Molar mass | 446.555 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
Erdafitinib, sold under the brand name Balversa, is a medication used to treat urothelial cancer.[2] Specifically it is used for cases which have suitable FGFR-2 or 3 mutations.[2] It is taken by mouth.[2]
Common side effects include high phosphate, mouth inflammation, tiredness, kidney problems, diarrhea, liver problems, change in taste, low magnesium, hair loss, high calcium, and muscle pain.[2] Other side effects may include infertility and vision problems.[2] Use in pregnancy may harm the baby.[2] It is a tyrosine kinase inhibitor, specifically a fibroblast growth factor receptor (FGFR) blocker.[1][2]
Erdafitinib was approved for medical use in the United States in 2019.[2] It is not approved for use in the United Kingdom or Europe as of 2021.[1] In the United States 4 weeks of treatment costs about 21,500 USD as of 2021.[3]
References[edit]
- ^ a b c "Erdafitinib". SPS - Specialist Pharmacy Service. 5 October 2018. Archived from the original on 11 January 2022. Retrieved 15 December 2021.
- ^ a b c d e f g h i j k "Erdafitinib Monograph for Professionals". Drugs.com. Archived from the original on 3 January 2022. Retrieved 15 December 2021.
- ^ "Erdafitinib Prices, Coupons & Savings Tips - GoodRx". GoodRx. Retrieved 15 December 2021.
