User:Mr. Ibrahem/Dexmethylphenidate
 | |
 | |
| Clinical data | |
|---|---|
| Trade names | Focalin, Focalin XR, Attenade, others |
| Other names | d-threo-methylphenidate (D-TMP) |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a603014 |
| License data | |
| Dependence liability | Physical: None Psychological: High |
| Routes of administration | By mouth |
| Legal status | |
| Legal status |
|
| Pharmacokinetic data | |
| Bioavailability | 11–52% |
| Protein binding | 30% |
| Metabolism | Liver |
| Elimination half-life | 4 hours |
| Excretion | Kidney |
| Identifiers | |
| |
| Chemical and physical data | |
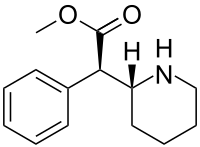
| Formula | C14H19NO2 |
| Molar mass | 233.311 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| | |
Dexmethylphenidate, sold under the brand name Focalin among others, is a medication used to treat attention deficit hyperactivity disorder (ADHD) in those over the age of 5 years.[3] If no benefit is seen after 4 weeks it is reasonable to discontinue its use.[3] It is taken by mouth.[3] The immediate release formulation lasts up to 5 hours while the extended release formulation lasts up to 12 hours.[4]
Common side effects include abdominal pain, loss of appetite, and fever.[3] Serious side effects may include abuse, psychosis, sudden cardiac death, mania, anaphylaxis, seizures, and dangerously prolonged erection.[3] Safety during pregnancy and breastfeeding is unclear.[1] Dexmethylphenidate is a central nervous system (CNS) stimulant.[5][3] How it works in ADHD is unclear.[3] It is the more active enantiomer of methylphenidate.[3]
Dexmethylphenidate was approved for medical use in the United States in 2001.[6] It is available as a generic medication.[3] The wholesale cost of a month supply in the United States is about US$8.[7] In 2017, it was the 189th most commonly prescribed medication in the United States, with more than three million prescriptions.[8][9] It is also available in Switzerland.[10]
References[edit]
- ^ a b "Dexmethylphenidate Use During Pregnancy". Drugs.com. Archived from the original on 15 April 2019. Retrieved 15 April 2019.
- ^ "WHOCC - ATC/DDD Index". www.whocc.no. Archived from the original on 25 January 2021. Retrieved 9 September 2020.
- ^ a b c d e f g h i "Dexmethylphenidate Hydrochloride Monograph for Professionals". Drugs.com. American Society of Health-System Pharmacists. Archived from the original on 7 November 2020. Retrieved 15 April 2019.
- ^ Mosby's Drug Reference for Health Professions - E-Book. Elsevier Health Sciences. 2013. p. 455. ISBN 9780323187602. Archived from the original on 2020-05-15. Retrieved 2019-04-15.
- ^ Moen MD, Keam SJ (December 2009). "Dexmethylphenidate extended release: a review of its use in the treatment of attention-deficit hyperactivity disorder". CNS Drugs. 23 (12): 1057–83. doi:10.2165/11201140-000000000-00000. PMID 19958043.
- ^ "DailyMed - dexmethylphenidate hydrochloride tablet". dailymed.nlm.nih.gov. Archived from the original on 7 August 2020. Retrieved 15 April 2019.
- ^ "NADAC as of 2019-02-27". Centers for Medicare and Medicaid Services. Archived from the original on 2019-03-06. Retrieved 3 March 2019.
- ^ "The Top 300 of 2020". ClinCalc. Archived from the original on 12 February 2021. Retrieved 11 April 2020.
- ^ "Dexmethylphenidate Hydrochloride - Drug Usage Statistics". ClinCalc. Archived from the original on 12 April 2020. Retrieved 11 April 2020.
- ^ "Focalin XR". Drugs.com. Archived from the original on 15 April 2019. Retrieved 15 April 2019.
