User:Mr. Ibrahem/Dabigatran
 | |
| Clinical data | |
|---|---|
| Trade names | Pradaxa, Pradax, Prazaxa, others |
| Other names | Dabigatran etexilate (USAN US) |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a610024 |
| License data |
|
| Pregnancy category |
|
| Routes of administration | By mouth |
| Legal status | |
| Legal status |
|
| Pharmacokinetic data | |
| Bioavailability | 3–7%[2] |
| Protein binding | 35%[2] |
| Elimination half-life | 12–17 hours[2] |
| Identifiers | |
| |
| Chemical and physical data | |
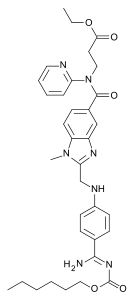
| Formula | C34H41N7O5 |
| Molar mass | 627.734 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| | |
Dabigatran, sold under the brand name Pradaxa among others, is an anticoagulant used to treat and prevent blood clots and to prevent stroke in people with atrial fibrillation.[2][4] Specifically it is used to prevent blood clots following hip or knee replacement and in those with a history of prior clots.[2] It is used as an alternative to warfarin and does not require monitoring by blood tests.[2] It is taken by mouth.[2]
Common side effects include bleeding and gastritis.[2] Other side effects may include bleeding around the spine and allergic reactions such as anaphylaxis.[2] In cases of severe bleeding, it can be reversed with the antidote, idarucizumab.[2] Use is not recommended during pregnancy or breastfeeding.[2] Compared to warfarin it has fewer interactions with other medications.[5] It is a direct thrombin inhibitor.[4]
Dabigatran was approved for medical use in the United States in 2010.[2] It is on the World Health Organization's List of Essential Medicines.[6] A month supply in the United Kingdom costs the NHS about £51 as of 2019.[4] In the United States the wholesale cost of this amount is about US$416.[7] In 2017, it was the 302nd most commonly prescribed medication in the United States, with more than one million prescriptions.[8]
References[edit]
- ^ a b "Dabigatran (Pradaxa) Use During Pregnancy". Drugs.com. 27 December 2018. Retrieved 16 May 2020.
- ^ a b c d e f g h i j k l "Dabigatran Etexilate Mesylate Monograph for Professionals". Drugs.com. American Society of Health-System Pharmacists. Retrieved 27 March 2019.
- ^ "WHOCC - ATC/DDD Index". www.whocc.no. Retrieved 8 September 2020.
- ^ a b c British national formulary : BNF 76 (76 ed.). Pharmaceutical Press. 2018. pp. 135–137. ISBN 9780857113382.
- ^ Kiser, Kathryn (2017). Oral Anticoagulation Therapy: Cases and Clinical Correlation. Springer. p. 11. ISBN 9783319546438.
- ^ World Health Organization (2019). World Health Organization model list of essential medicines: 21st list 2019. Geneva: World Health Organization. hdl:10665/325771. WHO/MVP/EMP/IAU/2019.06. License: CC BY-NC-SA 3.0 IGO.
- ^ "NADAC as of 2019-02-27". Centers for Medicare and Medicaid Services. Retrieved 3 March 2019.
- ^ "Dabigatran Etexilate Mesylate - Drug Usage Statistics". ClinCalc. Retrieved 11 April 2020.
