User:Mr. Ibrahem/Celecoxib
 | |
 | |
| Clinical data | |
|---|---|
| Pronunciation | /sɛlɪˈkɒksɪb/ SEL-i-KOK-sib |
| Trade names | Celebrex, Onsenal, Elyxyb, others |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a699022 |
| License data | |
| Pregnancy category |
|
| Routes of administration | By mouth |
| Drug class | NSAID (COX-2 inhibitor)[2] |
| Legal status | |
| Legal status | |
| Pharmacokinetic data | |
| Bioavailability | Unknown[4] |
| Protein binding | 97% (mainly to serum albumin)[4] |
| Metabolism | Liver (mainly CYP2C9)[4] |
| Elimination half-life | 7.8 hours; 11 hours (mild hepatic impairment); 13 hours (moderate-severe hepatic impairment)[4] |
| Excretion | Faeces (57%), urine (27%)[4] |
| Identifiers | |
| |
| Chemical and physical data | |
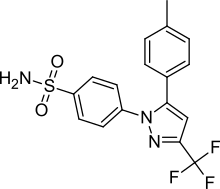
| Formula | C17H14F3N3O2S |
| Molar mass | 381.373 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| (verify) | |
Celecoxib, sold under the brand name Celebrex among others, is a COX-2 inhibitor and nonsteroidal anti-inflammatory drug (NSAID).[2] It is used to treat the pain and inflammation in osteoarthritis, acute pain in adults, rheumatoid arthritis, ankylosing spondylitis, painful menstruation, and juvenile rheumatoid arthritis.[2] It may also be used to decrease the risk of colorectal adenomas in people with familial adenomatous polyposis.[2] It is taken by mouth.[2] Benefits are typically seen within an hour.[2]
Common side effects include abdominal pain, nausea, and diarrhea.[2] Serious side effects may include heart attacks, strokes, gastrointestinal perforation, gastrointestinal bleeding, kidney failure, and anaphylaxis.[6][2] Use is not recommended in people at high risk for heart disease.[7][8] The risks are similar to other NSAIDs, such as ibuprofen and naproxen.[9] Use in the later part of pregnancy or during breastfeeding is not recommended.[2][1]
Celecoxib was patented in 1993 and came into medical use in 1999.[10] It is available as a generic medication.[11] A month supply in the United Kingdom costs the NHS less than £2 as of 2019.[11] In the United States, the wholesale cost of this amount is about $US12.90.[12] In 2011, it was one of Pfizer's best-selling medications, with $2.5 billion in sales.[13] In 2017, it was the 106th most commonly prescribed medication in the United States, with more than seven million prescriptions.[14][15]
References[edit]
- ^ a b c d "Celecoxib (Celebrex) Use During Pregnancy". Drugs.com. 4 May 2020. Archived from the original on 25 January 2021. Retrieved 5 May 2020.
- ^ a b c d e f g h i j k "Celecoxib Monograph for Professionals". Drugs.com. American Society of Health-System Pharmacists. 11 November 2019. Archived from the original on 20 May 2019. Retrieved 5 May 2020.
- ^ "Celebrex 100mg capsule - Summary of Product Characteristics (SmPC)". (emc). 13 January 2020. Archived from the original on 6 August 2020. Retrieved 5 May 2020.
- ^ a b c d e McCormack PL (December 2011). "Celecoxib: a review of its use for symptomatic relief in the treatment of osteoarthritis, rheumatoid arthritis and ankylosing spondylitis". Drugs. 71 (18): 2457–89. doi:10.2165/11208240-000000000-00000. PMID 22141388.
- ^ "WHOCC - ATC/DDD Index". www.whocc.no. Archived from the original on 2 July 2020. Retrieved 9 September 2020.
- ^ Bhala N, Emberson J, Merhi A, Abramson S, Arber N, Baron JA, Bombardier C, Cannon C, Farkouh ME, FitzGerald GA, Goss P, Halls H, Hawk E, Hawkey C, Hennekens C, Hochberg M, Holland LE, Kearney PM, Laine L, Lanas A, Lance P, Laupacis A, Oates J, Patrono C, Schnitzer TJ, Solomon S, Tugwell P, Wilson K, Wittes J, Baigent C (August 2013). "Vascular and upper gastrointestinal effects of non-steroidal anti-inflammatory drugs: meta-analyses of individual participant data from randomised trials". Lancet. 382 (9894): 769–79. doi:10.1016/S0140-6736(13)60900-9. PMC 3778977. PMID 23726390.
- ^ Antman EM, Bennett JS, Daugherty A, Furberg C, Roberts H, Taubert KA (March 2007). "Use of nonsteroidal antiinflammatory drugs: an update for clinicians: a scientific statement from the American Heart Association". Circulation. 115 (12): 1634–42. doi:10.1161/circulationaha.106.181424. PMID 17325246.
- ^ "Should you still take Celebrex?". Consumer Reports. August 2009. Archived from the original on 18 December 2015. Retrieved 27 December 2015.
- ^ Stein, Rob (25 April 2018). "FDA Panel Affirms Safety Of Painkiller Celebrex". NPR. Archived from the original on 20 May 2018. Retrieved 19 May 2018.
- ^ Fischer, Jnos; Ganellin, C. Robin (2006). Analogue-based Drug Discovery. John Wiley & Sons. p. 522. ISBN 9783527607495. Archived from the original on 28 August 2021. Retrieved 30 April 2020.
- ^ a b British national formulary : BNF 76 (76 ed.). Pharmaceutical Press. 2018. pp. 1097–1098. ISBN 9780857113382.
- ^ "NADAC as of 2019-02-27". Centers for Medicare and Medicaid Services. Archived from the original on 6 March 2019. Retrieved 3 March 2019.
- ^ Thomas, Katie (24 June 2012). "In Documents on Pain Drug, Signs of Doubt and Deception". The New York Times. Archived from the original on 3 January 2016. Retrieved 27 December 2015.
- ^ "The Top 300 of 2020". ClinCalc. Archived from the original on 12 February 2021. Retrieved 11 April 2020.
- ^ "Celecoxib - Drug Usage Statistics". ClinCalc. Archived from the original on 12 April 2020. Retrieved 11 April 2020.
