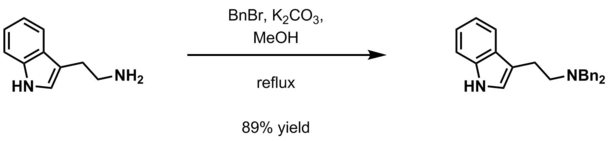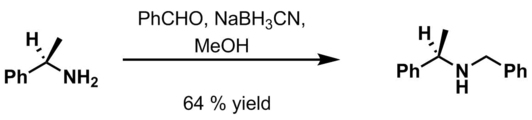User:Mpodunavac/sandbox
 | This is a user sandbox of Mpodunavac. You can use it for testing or practicing edits. This is not the sandbox where you should draft your assigned article for a dashboard.wikiedu.org course. To find the right sandbox for your assignment, visit your Dashboard course page and follow the Sandbox Draft link for your assigned article in the My Articles section. |
Amine Protection - Carboxybenzyl (Cbz)[edit]

Carboxybenzyl group (Cbz) is commonly used in organic synthesis as a protecting group for amines.
Most common amine protection methods[edit]
- Benzyl chloroformate and a base, such as sodium carbonate in water at 0°C[1]
- Benzyl chloroformate and magnesium oxide in ethyl acetate at 70°C to reflux[2]

- Benzyl chloroformate, DIPEA, acetonitrile and scandium trifluoromethanesulfonate (Sc(OTf)3)[3]

Most common amine deprotection methods[edit]
- Hydrogenation in the presence of the palladium catalyst[4]

Amine Protection - tert-butyloxycarbonyl (Boc)[edit]

Tert-butyloxycarbonyl (Boc) group is used as a protecting group for amines in organic synthesis.
Most common amine protection methods[edit]
- Heating a mixture of an amine and di-tert-butyl dicarbonate in tetrahydrofuran (THF) at 40 °C[5]
- Sodium hydroxide and di-tert-butyl dicarbonate in water at ambient temperature[6]
- di-tert-butyl dicarbonate, DMAP, and acetonitrile at ambient temperature[7]

Most common amine deprotection methods[edit]
Amine Protection - Fluorenylmethyloxycarbonyl (Fmoc)[edit]

Fluorenylmethyloxycarbonyl (Fmoc) group is used as a protecting group for amines in organic synthesis.
Most common amine protection methods[edit]
- Fluorenylmethyloxycarbonyl chloride or 9 -fluorenylmethyloxycarbonyl azide, sodium bicarbonate and aqueous dioxane[10]
Most common amine deprotection methods[edit]
- Tetra-n-butylammonium fluoride in the presence of dimethylformamide[11]
Amine Protection - Benzyl (Bz, Bn)[edit]

Benzyl (Bz, Bn) group is largely used as a protecting group for amines in organic synthesis.
Most common amine protection methods[edit]
- Aqueous potassium carbonate and benzyl halide (BnX; X=Cl, Br) in methanol[12]
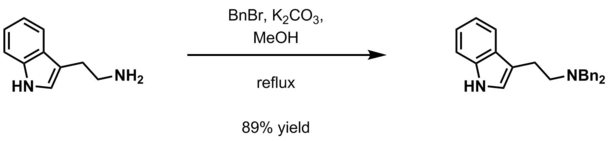
- Benzaldehyde, 6 M HCl and NaBH3CN in methanol[13]
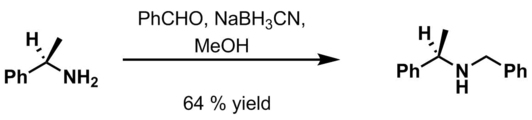
Most common amine deprotection methods[edit]
- Hydrogenation in the presence of the palladium catalyst[14]
Amine Protection - 2,2,2-Trichloroethyl (Troc)[edit]

2,2,2-Trichloroethyl (Troc) group is largely used as a protecting group for amines in organic synthesis.
Most common amine protection methods[edit]
- 2,2,2-Trichloroethyl chloroformate, pyridine or aqueous sodium hydroxide at ambient temperature[15]

Most common amine deprotection method[edit]
Amine Protection - Tosyl (Ts)[edit]

Tosyl (Ts) group is commonly used as a protecting group for amines in organic synthesis.
Most common amine protection methods[edit]
Most common amine deprotection methods[edit]
- HBr and acetic acid at 70 °C[16]

- Refluxing with TMSCl, sodium iodide and acetonitrile[17]
- ^ M. Bergmann and L. Zervas, Ber., 65, 1192 (1932)
- ^ Dymicky, M. (1989-02-01). "Preparation of Carbobenzoxy-L-Tyrosine Methyl and Ethyl Esters and of the Corresponding Carbobenzoxy Hydrazides". Organic Preparations and Procedures International. 21 (1): 83–90. doi:10.1080/00304948909356350. ISSN 0030-4948.
- ^ "A Formal Asymmetric Synthesis of()-Anatoxin-a Using an EnantioselectiveDeprotonation Strategy on an Eight-MemberedRing".
{{cite journal}}: Cite journal requires|journal=(help) - ^ Felpin, François-Xavier; Fouquet, Eric (2010-11-02). "A Useful, Reliable and Safer Protocol for Hydrogenation and the Hydrogenolysis of O-Benzyl Groups: The In Situ Preparation of an Active Pd0/C Catalyst with Well-Defined Properties". Chemistry – A European Journal. 16 (41): 12440–12445. doi:10.1002/chem.201001377. ISSN 1521-3765.
- ^ a b Wuts, Peter G. M.; Greene, Theodora W. Greene's Protective Groups in Organic Synthesis, Fourth Edition - Wuts - Wiley Online Library. doi:10.1002/0470053488.
- ^ Tarbell, D. Stanley; Yamamoto, Yutaka; Pope, Barry M. (1972-03-01). "New Method to Prepare N-t-Butoxycarbonyl Derivatives and the Corresponding Sulfur Analogs from di-t-Butyl Dicarbonate or di-t-Butyl Dithiol Dicarbonates and Amino Acids". Proceedings of the National Academy of Sciences. 69 (3): 730–732. ISSN 0027-8424. PMC 426545. PMID 16591972.
- ^ Englund, Ethan A.; Gopi, Hosahudya N.; Appella, Daniel H. "An Efficient Synthesis of a Probe for Protein Function: 2,3-Diaminopropionic Acid with Orthogonal Protecting Groups". Organic Letters. 6 (2): 213–215. doi:10.1021/ol0361599.
- ^ Stahl, Glenn L.; Walter, Roderich; Smith, Clark W. "General procedure for the synthesis of mono-N-acylated 1,6-diaminohexanes". The Journal of Organic Chemistry. 43 (11): 2285–2286. doi:10.1021/jo00405a045.
- ^ Prashad, Mahavir; Har, Denis; Hu, Bin; Kim, Hong-Yong; Girgis, Michael J.; Chaudhary, Apurva; Repič, Oljan; Blacklock, Thomas J.; Marterer, Wolfgang. "Process Development of a Large-Scale Synthesis of TKA731: A Tachykinin Receptor Antagonist". Organic Process Research & Development. 8 (3): 330–340. doi:10.1021/op0341824.
{{cite journal}}: no-break space character in|title=at position 58 (help) - ^ Carpino, Louis A.; Han, Grace Y. "9-Fluorenylmethoxycarbonyl amino-protecting group". The Journal of Organic Chemistry. 37 (22): 3404–3409. doi:10.1021/jo00795a005.
- ^ Farrera-Sinfreu, Josep; Royo, Miriam; Albericio, Fernando (2002-10-21). "Undesired removal of the Fmoc group by the free ε-amino function of a lysine residue". Tetrahedron Letters. 43 (43): 7813–7815. doi:10.1016/S0040-4039(02)01605-2.
- ^ Kuehne, Martin E.; Xu, Feng (1993-12-01). "Total synthesis of strychnan and aspidospermatan alkaloids. 3. The total synthesis of (.+-.)-strychnine". The Journal of Organic Chemistry. 58 (26): 7490–7497. doi:10.1021/jo00078a030. ISSN 0022-3263.
- ^ Cain, Christian M.; Cousins, Richard P. C.; Coumbarides, Greg; Simpkins, Nigel S. (1990-01-01). "Asymmetric deprotonation of prochiral ketones using chiral lithium amide bases". Tetrahedron. 46 (2): 523–544. doi:10.1016/S0040-4020(01)85435-1.
- ^ Zhou, Hao; Liao, Xuebin; Cook, James M. (2004-01-01). "Regiospecific, Enantiospecific Total Synthesis of the 12-Alkoxy-Substituted Indole Alkaloids, (+)-12-Methoxy-Na-methylvellosimine, (+)-12-Methoxyaffinisine, and (−)-Fuchsiaefoline". Organic Letters. 6 (2): 249–252. doi:10.1021/ol0362212. ISSN 1523-7060.
- ^ Marullo, N. P.; Wagener, E. H. (1969-01-01). "Structural organic chemistry by nmr. III. Isomerization of compounds containing the carbon-nitrogen double bond". Tetrahedron Letters. 10 (30): 2555–2558. doi:10.1016/S0040-4039(01)88566-X.
- ^ Haskell, Betty E.; Bowlus, Stephen B. (1976-01-01). "New synthesis of L-2-amino-3-oxalylaminopropionic acid, the Lathyrus sativus neurotoxin". The Journal of Organic Chemistry. 41 (1): 159–160. doi:10.1021/jo00863a042. ISSN 0022-3263.
- ^ Sabitha, Gowravaram; Reddy, B. V. Subba; Abraham, Sunny; Yadav, J. S. (1999-02-19). "Deprotection of sulfonamides using iodotrimethylsilane". Tetrahedron Letters. 40 (8): 1569–1570. doi:10.1016/S0040-4039(98)02646-X.