User:McMaster3F03/sandbox
The earliest Fossil record of Hominini appeared approximately 6mya, depicting a species with the ability of walking upright on two legs. With the expansion of grasslands bipedal hominins were able to find new sources of food, wild game, and utilize their bipedal walking to distinguish themselves from other hominoids. The change in locomotion allowed for several morphological changes to the skeleton, such as evolution of the forelimbs, Pelvis, feet, Skull, Chin, and Mandible. Additionally, hominins steadily developed a larger cranial capacity which resulted in a greater brain mass and level of intelligence. Other morphological changes, which currently exist in current human physiology, are no longer a necessity nor serve a substantial purpose. These vestigial traits include the appendix, Plantaris muscle, wisdom teeth, and Palmer Grasp Reflex.
Bipedalism[edit]
Bipedalism is a key Human Adaptation that reached a peak of evolution approximately 1.8 million years ago in Homo erectus.[1] Bipedalism is a form of locomotion that allows for the ability to walk upright on two legs. Several different hypotheses exist to help understand the contributions to the evolution of bipedalism.

Evolution[edit]
The adaptation to bipedalism was first marked approximately 5 to 6 million years ago. However, the process of becoming fully bipedal took longer than this, and was not perfected until the emergence of Homo erectus about 2 million years ago.[1] It was believed that walking upright with ease could have been a result of an increased brain size and consequently greater intelligence. This however is not the case as it is more recently proven that walking upright came well before the expansion in brain size.[1] When comparing humans to other quadraped mammals, they are not energetically efficient when it comes to high levels of locomotion such as running. Therefore, human bipedalism must have had other adaptive traits that allowed for its evolution.[2] The earliest hominid fossil remains came from East Africa, however very few of these fossils exist, which makes it more difficult to draw conclusions from these materials.[3] With that being said, other attempts must be made to reconstruct the evolution of bipedal locomotion. This can include indirect evidence such as comparison anatomy or behaviour of closely related ancestors such as apes.[1]
Origins of Bipedalism[edit]
Sexual Division of Labour[edit]
Male Partitioning Model[edit]
Selective pressures may have existed within monogamous pairs resulting in the evolution of bipedalism. The Male Provisioning Model suggests that the protection of food by males along with the carrying of the food could have resulted in bipedalism as an adaptable trait.[4]
The Carrying Hypothesis[edit]
As African land started to shift from dense forestry to more of a grassland area, food supplies began to dwindle. This lead to a change in how species retrieved their food, including the need for hauling and carrying food across larger distances. This phenomenon was first introduced by Owen Lovejoy, and is commonly known as The Carrying Hypothesis. This hypothesis coincides with the Male Partitioning Model, as they likely (along with several other factors) contributed to the evolution of bipedalism. This hypothesis arose from sexual selection and could have been a result of the need for males to carry food.[1] As food sources such as fruit trees became more scarce, greater distances needed to be traveled in order to obtain these resources essential to survival. This effect would come at a disadvantage to monogamous relationships, as females primary objective was to ensure the successful raising of offspring. In addition to the male's role of provisioning for his mate (See Male Provisioning Model), they were also responsible for carrying and transporting food.[1] The capability for males to carry food effectively meant that their upper limbs needed to be free, therefore favouring the ability for homininis to walk bipedally.[1]
Feeding Requirements[edit]
Postural Feeding Hypothesis[edit]
Hominini bipedalism is likely not just a result of adaptations to carrying food, but the act of retrieving food as well.[5] Adaptations made to bodily posture resulting in better food gathering is known as the Postural Feeding Hypothesis. The act of bipedal standing could have resulted in a species ability to reach higher into trees to gain food (specifically fruit).[5]The ability to gather food with both hands at little to no cost must have resulted in a quicker retrieval process, leading to the adaptive advantage of this posture.[5] Harvesting at a quicker rate would help speed up the process, as the gathering component of eating is the most time costly.[6] It is often believed that the evolution of bipedalism was more of a feeding adaptation rather than a locomotive one.[6]
Thermoregulatory Hypothesis[edit]
The locomotion of bipedalism could have resulted from hominins need for thermoregulation of the body, known as the Thermoregulatory Hypothesis. Heat balance models of thermoregulation are important for both the development of the upright bipedal stance, in addition to other factors such as body hair.[7]. The upright posture of bipedals is favourable when comparing it to a quadruped, especially in the terrestrial environments hominins evolved in.[8] Keeping the body cool was a factor of clear importance, as preventing overheating would enhance overall energy. In the hot savannah habits that these species resided in, a standing upright individual would be exposed to much less solar radiation than one who's body encompassed more surface area closer to the ground.[6] Not only this, but the location of the upper body in a bipedal organism means being subject to stronger convective wind, leading to faster cooling methods in extreme heat.[6] This effective method of heat loss likely played a large role in contributing to the adaptive advantage of bipedal locomotion in hominins.
Skeletal Structure[edit]
Within the hominin lineage there has been several skeletal structures that have changed throughout time that would allow for better locomotion, child birth, and food gathering. These changes can be seen starting with Australopithecines, other archaic forms and continue throughout the Homo lineage.
Forelimbs[edit]
The above theories suggest that as we started to become bipedal we used our arms for different tasks instead of locomotion. As a general consensus Australopithecines (Au.) are characterized by having large, long and robust arms compared to early Homo who had relatively petite arms.[9] Ardipithecus ramidus also had longer arm lengths and are characterized to be more ape like than modern humans today.[10] Au. afracanis, Au. garhi and Au. afarensis had relatively long arm lengths compared to leg lengths with the earlier form, Au. afracanis, being much larger than Au. afarensis.[9] The ratio of arms to legs gradually got smaller and then arrived at Homo where there was a drastic change and decrease in forelimb size where the legs are much longer than the arms.[9]
Pelvic Bone[edit]

Throughout the Hominin lineage there has also been many changes to the pelvic bone. Changes from the last common ancestor of Chimps and Humans (LCA-CH) have been linked to bipedalism.[9] Morphological changes within just the Homo lineage have occurred as well, which have been liked to reproduction and birth.[9] As we started to walk upright adaptations to the pelvis included: rotation of the ilium[11], shortening of the blades,[9] and anterior rotation of the sacrum.[9] Rotation of the ilium came about once we started to walk in a upright position.[11] Early Homo retained the flat structure and later adapted a rounder pelvic inlet which is present today in modern humans.[9] The shortening of the ilium lowers the center of mass and is evident is the skeletal remains of Lucy, or "Au. afarensis.[11] Although this structure is not exactly what we see today in modern humans, it may represent a transitional stage between being a quadruped to becoming bipedal. [12] This shortening of ilium also reduced the front to back dimensions of the birth canal in Au. afarensis which would have been okay for their smaller brains.[11] Today, in modern humans it has expanded from back to front which may allow for passage of newborns through birth canal.
Foot[edit]
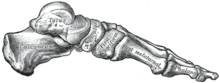
The foot contains shock absorbing arch that helps the body cope with the large loads of walking bipedal. [11] All the weight that was once distributed among four different limbs are not attributed to only two. Our ancestors had what is called an opposable toe which was used for grabbing whilst traveling around the trees. Ardipithecus (Ar.) ramidus still had the opposable toe but the rest of their toes were fit for walking upright.[10] Within our own species,Homo sapiens, the big toe is no longer opposable which is linked to the change in walking style to an upright position and is no longer used for grabbing or arboreal traveling that our ancestors would have experiences.[11] Au. afarensis is one of the lucky few that can contribute to the fossil record with long and curved toes.[11] In comparison, Homo ergaster fossils are said to be much shorter and much less curved. [11]
Skull and Cranial Capacity[edit]
One of the foremost adaptations was the cranial expansion of the skull cavity which resulted in an increased brain mass and level of intelligence. These changes occurred in earlier hominin ancestry and were the basis to which development of a mutual language, ability to craft tools and advances toward social interaction were capable.[13] By comparing the cranial capacity of several discovered fossils of early ancestors, there is clear indication of the increased proportions of the skull throughout the hominin lineage. One of the earliest hominin ancestors, the Australopithecus afarensis, had lived approximately 3-4 million years ago and had a cranial capacity of 350-450cm3. [14] It was a key species that indicated that bipedal locomotion preceded the evolutionary increase in brain size. [15] Afterwards, the Homo ¬genus arose and experienced the largest rate of increase in cranial capacity. Beginning with earliest member of the Homo genus, the Homo habilis, the cranial capacity ranged from 550-650 cm3. [14] Within that two million year period of the Homo habilis to today, the human species, Homo sapiens, have had an increase of about 500-1500 cm3 which resulted in the average cranial capacity increasing to a range of 1000-2000 cm3.[16] Throughout the 7 million years existence of the hominin lineage, the cranial capacity increased from 300cm3 to nearly 2000cm3. Some explanations and reasoning behind the expansion of the skull include causes such as climate change, social interaction and ecological dominance.
Theories[edit]
Climate Change[edit]
Frequent fluctuations in the climate resulted in many dynamic and varying habitats over time. These changes are theorized to have supported the adaptation and evolution of an increased cranial capacity. [17] Those individuals had several advantages towards survival and reproduction, many of which were successful in allowing longevity throughout a wide range of habitats and environmental conditions. [17] In order to survive the cold and warm shifts in climates, social interaction and cooperation as well as tools and shelter were necessary, which are all capable with an increased level of intelligence.[17] In addition, hominin species that migrated away from the equator would have faced the seasonal changes found at higher latitudes more frequently than those who remained in constant climatic conditions. Those variations would have resulted in the increased needed for larger cranial capacity required to adapt and face the inconsistent environments. [17] Therefore, both seasonal and yearly climate changes may be responsible for the varying cranial capacity.
Social Interaction[edit]
Survival and reproduction in large elaborate groups has suggested social interaction resulted in preferential adaption for an increased cranial capacity. Advanced behaviours such as reciprocation, deception and emotional response would serve as a fitness advantage in social groups. [18] Furthermore, complex groups with a larger number of members would promote increased intelligence towards managing and maintaining relationships due to the increased amount of individuals.[18] Social interaction may have been an important pressure toward sexual selection of a mate. Hominins did not have ornate horns or feathers to show fitness, instead may have relied on the ability to communication and interact. [19] Inter-species competition toward mate selection would be a contributing factor in evolutionary arms race in helping to provide a discernable advantage to the species as a whole. Every social exchange is unique to the last, as are the individuals whom are involved. Coupled with frequent and rapid relationship changes, this created the constantly shifting relationship conditions that would favor the evolution of cranial capacity. [19]
Ecological Dominance[edit]
Through evolution, hominin diet developed and included the consumption of meat, which could be increasingly successful with the ability to hunt using tools and cooperate with other individuals, which is indicative of increased mental systems. [19] The ability to craft and use tools would have aided in hunt success rate and provided elevated protection from predators and various competition. Projectile weapons, in particular, would require advanced motor skills necessary for tracking and aiming at moving targets, as well as dodging incoming weapons being thrown. [19] The ability to survive and dominate in the existing ecosystem proved to be beneficial with an evolutionary advantage of cranial capacity.
Facial Features[edit]
Morphological facial features and masticatory structures consisting of the maxilla, mandible, teeth and tempormandibular jaw have evolved, separating modern humans from living apes. The development of the above features has been carried out through extensive examination of cranial fossils relating to extinct hominins, as well as mechanical studies relating to the jaw and teeth of hominin taxa.[20] In addition to facial features involvement in speech, more importantly is their significant role in eating process.[20] Evidence dating back many years has shown a decrease in the size of the hominin masticatory system, leading to conclusions that the teeth and jaws of recent humans are smaller than those of great apes. Although only speculation, researchers believe that the decrease in size can be primarily attributed to changes in dietary intake. [20] Examining the dental and orofacial features of both modern humans and living apes, many distinguishing characteristics are observed.
Chin[edit]
One of the most salient maxillofacial features distinguishing modern humans from our common ancestors is a protruding chin. Although the functional importance of the chin is still in uncertain, researchers have concluded that this particular feature was absent in archaic humans. [20] Many theories have attempted to construe the formation of the human chin, some believing it to have evolved from biomechanical forces in the masticatory system or perhaps at a point in time when their was a decline in dental use and mandibular shortening. [20] Researchers, however, are consistent in their findings, confirming that the appearance of the protruding chin does in fact coincide with the emergence of speech around 50,000 years ago.[20]
Dentition and Mandable[edit]
The dietary capacity of early hominis has changed immensely, allowing them to survive in various habitats and leading to a shift in their tooth shape, size and dental biomechanics. [20] During the Pilocene period, studies suggest that their diet consisted of food that was hard and abrasive. Australopithecines, for example, have much larger molars than do today’s orangutan, therefore leading to much variation in tooth size. This provided them the ability to adapt to a variety of foods that differed in shape, size and texture. [20] Their diet consisted primarily of fruits, nuts, and flowers, with an anatomical disadvantage for the consumption of meat. Homo sapiens, however, have dentally adapted to consuming meat, with perhaps the use of tools for cutting and grinding dominating the need for a strong jawbone, large teeth and strong muscles. [20] Furthermore, the reduction in size of our jaw and teeth may be attributed to populations that rely more heavily on agriculture, rather than foraging for food. In order to explain the above reduction in jaw and teeth size, several models have been adapted and explored. The probable mutation effect seeks to describe this process by suggesting that mutations, in the absence of natural selection, will lead to a disruption of genetic mechanisms, resulting in a simplified dental structure. [20] In contrast, the increasing population density effect proposes the reduction of dental size is a result of a change in population due to a shift from a nomadic to sedentary lifestyle. From this, adaptive pressures changed, with a reduction in nutritional requirements and metabolic activity corresponding to a decrease in body size and consequently tooth size. [20] In addition to these two theories, a third model, the selective compromise effect implies that smaller teeth may have been selected due to dental crowding and high prevalence of disease. The subsequent dental reduction includes a change in overall crown area, with the hopes of decreasing the frequency of cavities. [20] Unlike the probable mutation effect model, the other two theories highlight the importance of selective forces as an explanation to the evolution of the human masticatory system. In 2008, Pinhasi et al. deemed the selective compromise effect model the most likely of the three to be true.[21]
Dentintion[edit]
The evolution of tooth reduction and cusp simplification seem to be the cause of natural selection, therefore we see long-term trends in the fossil record. Thorough examinations of the Villafranchian to Middle and then Upper Pleistocene populations confirm the consistent decrease in tooth dimensions are a result of direct selection.[22] Studies by Weidenreich proposed a decrease in jaw size and teeth could be attributed to the expansion of the brain, however, his results were not supported, therefore suggesting a reduction in dentition by the teeth themselves. In Pleistocene populations, the Australopithecus group favoured larger teeth given that they were an essential component responsible for chewing as well as tearing food and other materials.[22] As populations evolved, the use of tools increased dramatically and aided in tasks that were previously a function of dentition, further decreasing the need for a large jaw and teeth. [22] Dental differences in the incisors, canines and molars amoung hominin taxa have been observed and summarized in table 1[23], along with mandibular traits.[24] Evidence suggests that incisors were in the past much larger and sloped downwards, compared to smaller and vertically implanted, as seen in archaic megadont hominins and members of the genus Homo Reduction in size of incisors lead to the enlargement of pre-molars and molars.[24] Dating back to the earliest and archaic hominins, the size of the second molar tooth was generally seen as the largest. [20] Comparing that with modern humans, a slight difference is observed with the first molar tooth being considered the biggest in addition to a decrease in overall tooth size. In addition to these observed changes in dentition, the size of canine teeth were relatively small when dating back to the earliest hominins. Their seemed to have been a continual decrease in the size of canines, with the biggest reduction seen in the megadont archaic hominins.[24]
Mandable[edit]
The evolution of the genus Homo is correlated with the emergence of many defining features, one being the importance of masticatory (jaw) muscles.[20] The lower jaw muscle has been adapted over time in order to withstand certain stresses and strains placed on it by food processing, therefore confirming its close association with diet. [25] Comparing the mastication of hard and soft foods, experiments performed in the past decade on modern human subjects have shown that hard foods are correlated with a larger lateral excursion of the mandible. [24] Thus, it is possible to conclude that a hard diet requires a much wider and broader development of the mandible. [24] In addition, examination of the ratio between corpus breadth to height suggests that early hominins have a much thicker mandibular corpus, suggesting a morphological shift must have occurred. [25] As mentioned above, the unique shape of the corpus reflects elevated stress regarding mastication, further verifying a diet that reflects fibrous, course foods.[25]
Vestigial Traits & Past Environments[edit]
Looking at past species of homminin in comparison to the current Homo"" species, a vast disparity is seen. These disparities or differences are primarily due to the environment a respective hominin species encountered and adaptive traits that manifested due to the environments.[26] Since then, the average environment has changed drastically and many of these changes can still be found in Homo sapiens. These left over changes that have no use in current environments are known as vestigial traits.[27] Furthermore, other changes led to significant behaviours that helped develop the current Homo sapiens species.
Ear muscles[edit]
Ear muscles: As seen in species of Macaque, ear muscles are primarily used to find the source of sounds in the environment.[28] With the development of bipedalism came the ability to rotate the head.[8] This ability to rotate the head counter-acted the ability to move the ears, rendering the required muscles feckless.[8] This idea also lends into the current shape of ears; the pinna is typically shaped to help funnel sounds into the ear canal.[28] However, with the development of head rotation and the loss of functioning ear muscles, the pinna became flatter and smaller in comparison to other species as it is compensated from bipedalism and head rotation, as many mammals that cannot turn their head have much larger pinna on their ears to help collect the sound of their environment.[28]

Appendix[edit]
An organ that used to hold digestive functions, possibly for the hydrolysis of cellulose and other indigestible floral materials; some believe it has developed a different function to help prevent the loss of symbiotic bacterial colonies residing in the intestinal tract.[29] The current leading theory in the derivation of the vermiform appendix is that it is the remnants of the once much larger cecum. [30] The cecum is found at the beginning of the large intestine of the gastrointestinal tract. The appendix would have been connected to the cecum to help in processing fecal matter and diets rich in plant foliage.[27] As diets changed to foods that were more easily digestible, this allowed deleterious mutations that would normally have led people to be at a disadvantage, no longer cause a detrimental effect.[31] This allowed the shrinkage of the cecum to be tolerable, leading the appendix to be no longer needed for digestion.[26]
Plantaris Muscle[edit]
Primarily used for the movement of the ankle joint and flexion of the knee; derived from the habits of the hunter-gatherer societies when chasing game for numerous kilometres.[29] The original function of the muscle was most likely to enable grasping objects with the feet before bipedalism evolved into the hominini populations.[29] However, as the foot structure changed with bipedalism, the muscle adapted to help with flexion of the ankle and knee for this type of walking.[9] On the other hand, due to the drastic change that occurred, the evolution of the leg structure rendered this muscle relatively useless, reasoning why it is not found in approximately 10% of the population.
Wisdom teeth[edit]
The wisdom teeth were a set of molars that helped with the chewing and grinding of food.[26] Before bipedalism developed, the main method of capturing prey would have been the use of teeth. [32] Furthermore, the early species of hominin had larger jaws to support the arrival of this extra set of molars.[31] However, as diet, environmental pressures and facial features changed, the requirement for this extra set of molars was no longer needed, nor is it no longer supported as the jaw has undergone change and become smaller.[9]
Cutis Anserina[edit]
During times of cold environments or situations that may allude to emotions of fear, pleasure, euphoria or arousal, the body will develop what is commonly known as “goose bumps”. This reflex, known as the pilomotor reflex, is present in many relatives to the hominini tribes, as well as the ancestors of Homo sapiens.[9] Many of Homo sapiens ancestors had much larger amounts of hair, which would make the pilomotor reflex useful.[8] As the “goose bumps” developed from cold environments, it would increase the surface area of the skin and trap air and provide insulation for the animal, allowing it to stay warm in cold conditions with the use of the arrector pili muscles to raise the hair follicles along the epidermis.[8] In response to fear, the arrector pilli muscles would become active and raise the hair follicles of the species, causing the animal to appear much larger in order to scare away its predators. In current Homo sapiens, this reflex and muscle have very little function, as humans today do not have the amount of hair required to make use of this reflex, deeming it a vestigial trait.[8]
Palmer Grasp Reflex[edit]
When a child is born, an inept ability to grasp things place in its hand is known as the Palmar Grasp Reflex.[33] In today’s society, infants are coddled for a relatively long time until they are able to look after themselves to a certain extent. This reflex is most likely the remnants of past environments where hominini species would have required this reflex.[33] The reflex is deemed as having the potential of being able to hold the infant’s own weight.[33] Before bipedalism, this trait would have proved useful, as it does for many chimpanzees, for the infant to hold on to the mother as she travel.[9] As bipedalism developed, the arms were able to hold the infant, thus nullifying the use of this reflex for traveling purposes.[33]
References[edit]
- ^ a b c d e f g Friedman, J. (2006). The Evolution of Hominid Bipedalism. Honors Projects. Paper 16. Retrieved from http://digitalcommons.iwu.edu/socanth_honproj/16
- ^ Rodman, P., & McHenry, H. (1980). Bioenergetics and the origin of hominid bipedalism. American Journal of Physical Anthropology 52: 103-106. Retrieved from: http://courses.biology.utah.edu/bramble/5360/readings/Rodman_80.pdf
- ^ Langdon, J. (1985). Fossils and the origin of bipedalism. Journal of Human Evolution 14: 615-635. Retrieved from: http://www.sciencedirect.com/science/article/pii/S0047248485800713
- ^ Lovejoy C. O. (1981). Male provisioning model. Retrieved from http://www.waterside-hypotheses.com/UploadedFiles/Wading Paper/Supporting Files/model/s1_2_4.html
- ^ a b c Richmond, Brian G.; Begun, David R.; Strait, David S. (2001). "Origin of human bipedalism: The knuckle-walking hypothesis revisited". American Journal of Physical Anthropology. 116 (S33): 70–105. doi:10.1002/ajpa.10019. PMID 11786992.
- ^ a b c d Hunt, K. (1994). The evolution of human bipedality: ecology and functional morphology. Journal of Human Evolution, 26 (3): 183–202. Retrieved from http://www.sciencedirect.com/science/article/pii/S0047248484710116
- ^ Ruxton, Graeme D.; Wilkinson, David M. (2011). "Avoidance of overheating and selection for both hair loss and bipedality in hominins". Proceedings of the National Academy of Sciences. 108 (52): 20965–20969. doi:10.1073/pnas.1113915108. PMC 3248486. PMID 22160694.
- ^ a b c d e f Wheeler, P. E. (1984). The Evolution of Bipedality and Loss of Functional Body Hair in Hominoids." Journal of Human Evolution 13: 91-98. Cite error: The named reference "Wheeler" was defined multiple times with different content (see the help page).
- ^ a b c d e f g h i j k l McHenry, H. and Coffing, K. (2000). Australopithecus to Homo: Transformation of Body and Mind. Annu. Rev. Anthropol 29: 125-146. Retrieved from : http://www.jstor.org/stable/223418
- ^ a b Boyd, R., and Silk, B. (2012). How Humans Evolved. Custom Edition McMaster University. In Biology 1M03(January 2013 ed.). Hamilton, ON: McMaster University.
- ^ a b c d e f g h Lovejoy, C. O. (1988). Evolution of Human Walking. "Scientific American," 118-125. Retrieved from: http://www.clas.ufl.edu.libaccess.lib.mcmaster.ca/users/krigbaum/proseminar/Lovejoy_1988_SA.pdf
- ^ Mednick, L. (2005). The Evolution of the Human Ilium. Am. Journal of Physical Anthropol. 13(2): 203-216. DOI: 10.1002/ajpa.1330130204
- ^ Bailey, D. H., and Geary, D. C. (2009). Hominid brain evolution: Testing climatic, ecological, and social competition models. Human Nature 20(1): 67-79.
- ^ a b Holloway, R.L. (1973). Endocranial volumes of early African hominids and the role of the brain in human mosaic evolution. Journal of Human Evolution 2: 449-459.
- ^ Falk, D. (1990). Brain Evolution in Homo: The “radiator” therory1. Behavioral and Brain Sciences 13(2): 333-334.
- ^ Leigh, Steven R. (1992). "Cranial capacity evolution in Homo erectus and early Homo sapiens". American Journal of Physical Anthropology. 87 (1): 1–13. doi:10.1002/ajpa.1330870102. PMID 1736667.
- ^ a b c d Ash, Jessica; Gallup, Gordon G. (2007). "Paleoclimatic Variation and Brain Expansion during Human Evolution". Human Nature. 18 (2): 109–124. doi:10.1007/s12110-007-9015-z. PMID 26181844. S2CID 580878.
- ^ a b Dunbar, R.I.M. (2003). "T S B : Mind, Language, and Society in Evolutionary Perspective". Annual Review of Anthropology. 32 (1): 163–181. doi:10.1146/annurev.anthro.32.061002.093158.
- ^ a b c d Flinn, Mark V.; Geary, David C.; Ward, Carol V. (2005). "Ecological dominance, social competition, and coalitionary arms races". Evolution and Human Behavior. 26 (1): 10–46. doi:10.1016/j.evolhumbehav.2004.08.005.
- ^ a b c d e f g h i j k l m n Emes, Y et al. (2011). On the evolution of human jaws and teeth: A review. Bulletin of the International Association of Paleodontology, 5(1): 37-47. Retrieved from http://ojs.sfzg.hr/index.php/IAPO/article/view/98/49
- ^ Pinhasi, R., Eshed V., Shaw, P. (2008). Evolutionary Changes in the Masticatory Complex Following the Transition to Farming in the Southern Levant. American Journal of Physical Anthropolology, 135(2): 136–148. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/18046779
- ^ a b c Greene, D. L. (1970). Environmental influences on pleistocene hominid dental evolution. BioScience 20(5): 276-279. Retrieved from http://www.jstor.org/stable/1295205
- ^ Table 1. Retrieved from"http://www.ncbi.nlm.nih.gov/pmc/articles/PMC2409106/table/tbl1/
- ^ a b c d e Lucas, P. Q., Constantino, P. J., & Wood, B. A. (2008). Inferences regarding the diet of extinct hominins: structural and functional trends in dental and mandibular morphology within the hominin clade. Journal of Anatomy, 212(4): 486-500. doi: 10.1111/j.1469-7580.2008.00877. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/18380867
- ^ a b c Ungar, P. Q., & Teaford, M. (n.d.). A paleontological perspective on the evolution of human diet. Informally published manuscript, Department of Anthropology, University of Arkansas, Fayetteville, AR. Available from Williamsburg, International Congress of Anthropological and Ethnological Studies. Retrieved from http://www.cast.uark.edu/local/icaes/conferences/wburg/posters/pungar/satalk.htm
- ^ a b c Leonard, W. R. (2002). Dietary change was a driving force in human evolution. Scientific American, 288: 63-71.
- ^ a b GRIFFITHS, PAUL E. (1993). "Functional Analysis and Proper Functions". The British Journal for the Philosophy of Science. 44 (3): 409–422. doi:10.1093/bjps/44.3.409.
- ^ a b c Moore, K. L., & Dalley, A. F. (1999).Clinically oriented anatomy (4th ed.). Philadelphia: Lippincott Williams & Wilkins.
- ^ a b c Aiello, L., & Dean, C. (1990). An introduction to human evolutionary anatomy. London: Academic Press.
- ^ Randal Bollinger, R., Barbas, A. S., Bush, E. L., Lin, S. S., & Parker, W. (2007). Biofilms in the large bowel suggest an apparent function of the human vermiform appendix. Journal of theoretical biology, 249(4): 826-831.
- ^ a b Teaford, Mark F.; Ungar, Peter S. (2000). "Diet and the evolution of the earliest human ancestors". Proceedings of the National Academy of Sciences. 97 (25): 13506–13511. doi:10.1073/pnas.260368897. PMID 11095758.
- ^ Walker, A. (1981). "Dietary Hypotheses and Human Evolution". Philosophical Transactions of the Royal Society B: Biological Sciences. 292 (1057): 57–64. doi:10.1098/rstb.1981.0013. PMID 6115407.
- ^ a b c d RICHTER, CURT P. (1931). "The Grasping Reflex in the New-Born Monkey". Archives of Neurology and Psychiatry. 26 (4): 784. doi:10.1001/archneurpsyc.1931.02230100102008.

