User:Materialsgrp/sandbox
Polymer Separators
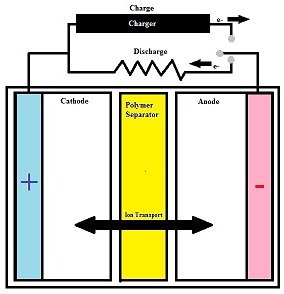
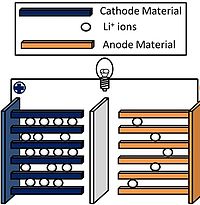
A polymer separator is a permeable membrane placed between the anode and cathode of a battery. The main function of a separator is to keep the positive and negative electrodes, the cathode and anode respectively, apart to prevent electrical short circuits while also allowing the transport of ionic charge carriers which are needed to complete the circuit during the passage of current in an electrochemical cell.[1]
Background[edit]
Polymer separators are critical components in liquid electrolyte batteries. The separator is placed between the positive and negative electrode in order to prevent physical contact of the electrodes while enabling ionic transport. They are generally comprised of a microporous layer consisting of a polymeric membrane. The separator must be chemically and electrochemically stable towards the electrolyte and electrode materials, while also being mechanically strong enough to withstand the high tension of battery construction. They are important to batteries because their structure and properties considerably affect the battery performance, including the batteries energy and power densities, cycle life, and safety.[2]
History[edit]
Unlike many forms of technology, polymer separators were not developed specifically for batteries. They were instead a result of spin-offs of existing technologies, which is why most polymer separators are not optimized for many of the systems they are used in. Even though this may seem unfavorable, most polymer separators can be mass produced at a comparatively low cost, because they are based on existing forms of technologies. [3] Dr. Yoshino et al. of the Asahi Kasei Corporation first developed a prototype of secondary lithium-ion batteries (LIBs) in 1983.
These prototype rechargeable cells included two electrodes; the cathode and anode. Initially, lithium cobalt oxide was used as the cathode and polyacetylene as the anode. Later in 1985, it was found that using lithium cobalt oxide as the cathode and graphite as the anode produced an excellent secondary battery based on both enhanced battery stability and the frontier electron theory of Dr. Kenichi Fukui [4] This enabled the development of portable equipment, such as cell phones and laptops. However, before lithium ion batteries could be mass produced for widespread use, safety concerns needed to be addressed such as overheating and over potential. One key to ensuring safety has been the use of a separator between the cathode and anode. This prevents physical contact between the two electrodes while still enabling ionic transport. Furthermore, Dr. Yoshino developed a microporous polyethylene membrane separator with a “fuse” function. [5] In the case of abnormal heat generation within the battery cell, the separator provides a shutdown mechanism in which the micropores of the separator close by melting and the ionic flow instantly terminates. In 2004, a novel electroactive polymer separator with the function of overcharge protection was first proposed by Dr. Denton et al. [6] This kind of separator can switch reversibly between insulating and conducting states in response of the changes in charge potential based on the intrinsic properties of the conducting polymer. Therefore, one can see how polymer separator’s function and purpose has changed over time. Now, a separators primary function is to provide a protection mechanism for a battery while also effectively transporting ionic charge carriers between the two electrodes as well as preventing the electric contact between them.
Synthesis[edit]
Polymer separators generally fall in the category of microporous polymer membranes. Microporous polymer membranes are usually fabricated from a variety of inorganic, organic, and naturally occurring materials. The pore size in these types of polymer separators is typically larger than 50-100 Å. Materials such as nonwoven fibers (cotton, nylon, polyesters, glass), polymer films (polyethylene, polypropylene, poly (tetrafluoroethylene), poly (vinyl chloride), and naturally occurring substances (rubber, asbestos, wood). There are also ion exchange membranes which are fabricated from polymeric materials that have pores with diameters of less than 20 Å. These are not typically used in batteries because their pore size is too small. The methods for manufacturing the microporous membranes and ion exchange membranes can be divided into two processes: dry process and wet processes. [7] [8]
Dry Process[edit]
The dry process is comprised of three steps: extruding, annealing, and stretching. The extruding step is generally carried out at a temperature higher than the melting point of the polymer resin. This is because the polymer resins are melted in order to shape them into a uniaxially orientated tubular film, called a precursor film. The structure and orientation of the precursor film produced depends on the processing conditions and the characteristics of the polymer resin used. In the next step, the annealing process, the precursor polymer is annealed at a temperature slightly lower than the melting point of the polymer. The purpose of this step is to improve the crystalline structure in order to enable the formation of micropores in the final step, stretching. In the final step, stretching, the annealed film is deformed along the machine direction by a process consisting of a cold stretch, a hot stretch, and a relaxation. The cold stretch is used to create the pore structure by stretching the film at a lower temperature with a faster strain rate, and the hot stretch is to increase the size of the pores by further stretching the film at a higher temperature with a slower strain rate. The purpose of the relaxation step is to reduce internal stress within the film. The porosity of the final film depends on the morphology of the precursor film, annealing conditions, and the stretching ratios and conditions. [9] [10]
Wet Process[edit]
Similar to the dry process the wet process consists of three steps: the mixing of the polymer resins, paraffin oil, antioxidant and other additives and then heating to produce a homogenous solution, then forcing the heated solution through a sheet die into a gel-like film, and then finally extracting the paraffin oil and other additives with a volatile solvent to form the microporous structure. [11]
Different types of polymers used in batteries[edit]
There are specific types of polymers which are ideal for the different types of synthesis. Most of polymers currently used in battery separators are polyolefin based materials with semi-crystalline structure. Among them, polyethylene, polypropylene, and their blends such as polyethylene-polypropylene are widely used. Recently, graft polymers have been studied in an attempt to improve battery performance, including micro-porous poly(methyl methacrylate)-grafted [12] and siloxane grafted polyethylene separators, which show favorable surface morphology and electrochemical properties as compared to conventional polyethylene separators. In addition, poly(vinylidene fluoride) (PVDF) nanofiber webs can be synthesized as a separator to improve both ion conductivity and dimensional stability [13] Another type of polymer separator, polytriphenylamine (PTPAn)-modified separator, is an electroactive separator with reversible overcharge protection. [6]
Ideal Polymers for Dry Processes[edit]
-
Structure of Polypropylene
The dry process is only suitable for polymers with high crystallinity. These include but are not limited to: semi-crystalline polyolefins, polyoxymethylene, and isotactic poly (4-methyl-1-pentene). One can also use blends of two immiscible polymers, in which at least one polymer has a crystalline structure, such as polyethylene-polypropylene, polystyrene-polypropylene, and poly (ethylene terephthalate) - polypropylene blends. [14] [15]
-
Structure of Polyethylene
Ideal Polymers for Wet Processes[edit]
The wet process is suitable for both crystalline and amorphous polymers. The separators synthesized by wet processes often use ultrahigh-molecular-weight polyethylene. The use of these polymers enables the batteries to have favorable mechanical properties while also preventing the battery from functioning when it becomes too hot. [16]
Wet process vs. Dry process[edit]
Membranes synthesized by dry processes are more suitable for a high power density battery because they have an open and uniform pore structure, while those made by wet processes are more suited for a long cycle life battery because of their tortuous and interconnected porous structure. This helps to suppress the growth of Li crystals on the graphite anode during fast charging or low temperature charging.[17]
Placement of Polymer Separators in Batteries[edit]
The separator is placed between the anode and the cathode. The pores of the separator are filled with the electrolyte. The electrode and separator combination is then wound into tight rolls which are then fitted into rigid cylindrical or prismatic (rectangular) metal cans. [18]
Properties of Polymer Separators[edit]
Chemical Stability[edit]
The separator material must be chemically stable against the electrolyte and electrode materials, especially under the strongly reductive and oxidative environments when the battery is fully charged. The separator should not degrade and lose mechanical strength. One can determine the chemical stability of a polymer separator by calendar life testing. [16]
Thickness of Separator[edit]
A battery separator must be relatively thin in order to facilitate the high energy and power densities of the battery. However, if the separator is too thin, it can decrease the mechanical strength and safety of the battery. Additionally, a separator should have uniform thickness in order to support the long like cycle of a battery. In current technologies, 25.4μm is generally accepted as the standard width. The thickness of a polymer separator can be measured using the T411 om-83 method developed under the auspices of the Technical Association of the Pulp and Paper Industry. [19]
Porosity of Separator[edit]
The separator must have the correct amount of porosity in order to hold a sufficient amount of liquid electrolyte in order to enable the movement of ions between the electrodes. The porosity cannot be too high because this hinders the ability of the pores to close, which is a vital component of the separators ability to shut down a battery. The porosity can be measured using liquid or gas absorption methods according to the American Society for Testing and Materials (ASTM) D-2873. Typically, a Li-ion battery separator will have a porosity of 40%. [17]
Pore Size[edit]
Pore size is also very important to the functioning of the separator. The pore size must be smaller than the particle size of the electrode components, including the electrode active materials and the conducting additives. Ideally the pores should be uniformly distributed while also having a tortuous structure. This ensures a uniform current distribution throughout the separator while suppressing the growth of Li on the anode. The distribution and structure of pores can be analyzed using a Capillary Flow Porometer or a Scanning Electron Microscope. [20]
Permeability[edit]
The separator should not limit the electrical performance of the battery. Usually the presence of a polymer separator will increase the resistance of the electrolyte by a factor of four to five. The ratio of the resistance of the separator filled with electrolyte divided by the resistance of the electrolyte alone is called the MacMullin number. Air permeability can be used indirectly to estimate the MacMullin number. Air permeability is expressed in terms of the Gurley value, which is defined as the time required for a specific amount of air to pass through a specific area of the separator under a specific pressure. The Gurley value reflects the tortuosity of the pores, when the porosity and thickness of the separators are fixed. A separator with uniform porousness is vital to the long life cycle of a battery. Deviations from uniform permeability will result in uneven current density distribution, which causes the formation of Li crystals on the graphite anode.[21] [22]
Mechanical Strength[edit]
The separator must be strong enough to withstand the tension of the winding operation during battery assembly. The mechanical strength of the polymer separator is also very important. Mechanical strength is typically defined in terms of the tensile strength in two directions, the machine direction and the transverse direction, and terms of the tear resistance and puncture strength. All of these parameters are defined in terms of Young’s modulus. [23]
Wetability[edit]
The electrolyte must be able to fill the entire battery assembly therefore, it is important that the separator wet easily when submerged in the electrolyte. Furthermore, the separator should be able to retain the electrolyte permanently, which increases the cycle life of the battery. There is not a generally accepted method used to test wettability, other than placing a droplet of electrolyte onto the separator and observing what happens. [24]
Stability[edit]
It is important that the separator remain stable over a wide temperature range. It is essential that once the separator is soaked with electrolyte it lays completely flat. [25]
Thermal Capabilities[edit]
Another major requirement for separators in lithium-ion batteries is the ability to shut down at a temperature slightly lower than that at which thermal runaway occurs. Even though the separator must be able to shut down at particular temperatures, it must be able to retain its mechanical properties. [26]
Defects[edit]
Many Structural defects can form in polymer separators due to temperature changes. These structural defects can result in a thicker separators. Furthermore, there can be intrinsic defects in the polymers themselves, such as polyethylene often begins to deteriorate during the stages of polymerization, transportation, and storage. [27] Additionally, defects such as tears or holes can form during the synthesis of polymer separators. There are also other sources of defects can come from doping the polymer separator.[2] Recently groups have been trying to improve the wetability of the polyer separators by co-dopping the normal polyethylene separator with acrylonitrile. The researchers found that acrylonitrile was more susceptible to be compatible with the electrolyte due to the wettability property. [28]
Use in Li-ion Batteries[edit]
Polymer separators, similar to battery separators in general, act as a separator of the anode and cathode in the Li-ion battery while also enabling the movement of ions through the cell. Additionally, many of the polymer separators, typically multilayer polymer separators, can act as “shutdown separators”, which are able to shut down the battery if it becomes too hot during the cycling process. These multilayered polymer separators are generally comprised of one or more polyethylene layers which serve to shut down the battery and at least one polypropylene layers which acts as a form of mechanical support for the separator. [6] [29]
Other types of battery separators[edit]
In addition to polymer separators, there are several other types of separators. There are nonwovens, which are comprised of a manufactured sheet, web, or matt of directionally or randomly oriented fibers. Supported liquid membranes, which consist of a solid and liquid phase contained within a microporous separator. Additionally there are also polymer electrolytes which can form complexes with different types of alkali metal salts, which results in the production of ionic conductors which serve as solid electrolytes. Another type of separator, a solid ion conductor, can serve as both a separator and the electrolyte in a battery. [30]
Advancements in Polymer Separators[edit]
The topic of polymer separators has been an active area of research due to the application of lithium-ion batteries in full or hybrid electric vehicles. These types of vehicles need to have lithium-ion batteries that contain high energy and power density. In other words, if one were to accelerate a full electric vehicle the secondary cell needs to output a large amount of energy as quickly as possible. DupontTM has introduced a novel nano-fiber based polymeric battery separator that boosts the performance and safety of lithium-ion batteries. These types of separators, named EnergainTM, are potential candidates for use in full electric vehicles in the near future. [31] The EnergainTM separators are synthesized into a web using a proprietary spinning process that creates continuous filaments. These filaments can range in diameter from 200 – 1,000 nanometers. The separators exhibit stability and low shrinkage in high temperatures and are easily saturated in common organic electrolytes. This results in more efficient operation, longer battery life, and improved safety. Batteries containing this type of separator can be quickly recharged, deliver improved performance, and reduce the number of cells needed by up to thirty-three percent for hybrid electric vehicles. Overall, this type of polymer separator can increase power up to thirty percent. Also, the battery life can also be increased by twenty percent. This is due to their stability at high temperatures and the overall morphology of the separator. [32] With more battery power, drivers can travel farther on a single charge and accelerate more quickly and safely.
As seen previously, polymer separators are of great importance, especially in the area of lithium-ion batteries. Jun Young Kim at Massachusetts Institute of Technology used plasma technology to modify a polyethylene membrane to create a high performance separator for practical applications in rechargeable lithium ion polymer batteries. Plasma treatment methods have been developed to modify polymer surfaces for enhanced adhesion, wettability, and printability. These are usually performed by modifying the surfaces on only several molecular levels. This allows the surface functionalization of polymers without sacrificing the bulk properties. The surface of the polyethylene membrane was modified with acrylonitrile via plasma coating technique. The lithium-ion polymer cell that contained the plasma induced acrylonitrile coated polyethylene (PiAn-PE) membrane was analyzed using various spectroscopic techniques. The surface characterization demonstrated that the enhanced adhesion of PiAN-PE membrane resulted from the increased polar component of surface energy. The presence of PiAN induced onto the surface of PE membrane via plasma modification process plays a crucial role in improving the wettability and electrolyte retention, the interfacial adhesion between the electrodes and the separator, and the cycle performance of the resulting lithium-ion polymer cell assembly. [33] This plasma-modified PE membrane holds a great potential to be a promising polymer membrane as a high-performance and cost-effective separator for lithium-ion polymer batteries. These polymer separators are also used in other secondary cells.
Another example of a secondary cell is the sealed rechargeable nickel/metal hydride battery. This offers significant improvement over conventional rechargeable batteries in terms of performance and environmental friendliness. The Ni/MH, like the lithium-ion battery, has the ability to display high energy and power density. These batteries have long cycle lives making them a leading technology as a battery source for electric vehicles. However, the greatest problem of Ni/MH cells are their inherent high corrosion rate in aqueous solutions. As a contribution to alkaline Ni/MH secondary battery technology, there has been a strong demand to replace the conventional aqueous electrolyte by a solid or gel polymer electrolyte/separator. In Ni/MH cells, the most commonly used separators are porous insulator films of polyolefin, nylon, or cellophane. Another way to modify these porous insulator films is the process of radiation grafting. Acrylic compounds can be radiation-grafted onto these separators to make their properties more desirable i.e. more wettable and permeable to the electrolyte. Zhijiang Cai and co-workers developed a solid polymer membrane gel separator. This comprised of a polymerization product of one or more monomers selected from the group of water-soluble ethylenically unsaturated amides and acid. The polymer-based gel also includes a water swellable polymer, which acts as a reinforcing element. In addition, ionic species are added to the solution and remain embedded in the polymer gel after polymerization. Recently, more and more Ni/MH batteries of bipolar design are being developed because they offer some advantages for applications as high power storage systems for electric vehicles. It was found that this solid polymer membrane gel separator could be very useful for such applications in bipolar design. In other words, this design can help in avoiding short-circuits occurring in liquid-electrolyte systems. [34]
Inorganic polymer separators have also been of interest as use in lithium-ion batteries. Inorganic particulate film/poly(methyl methacrylate) (PMMA)/inorganic particulate film trilayer separators are prepared by means of simple dip-coating of inorganic particle layers on to both sides of PMMA thin films. This inorganic trilayer membrane is believed to be an inexpensive, novel separator for application in lithium-ion batteries due to the increased dimensional and thermal stability. [35]
References[edit]
- ^ Flaim, Tony, Yubao Wang, and Ramil Mercado. "High Refractive Index Polymer Coatings." SPIE Proceedings of Optical Systems Design. Web
- ^ a b Arora, Pankaj and Zhang, Zhengming (John). “Battery Separators” Chemical Reviews 2004 104 (10), 4419-4462
- ^ Choi, Sung-Seen, Soo Lee, Young, Whan Joo, Chang Goo Lee, Seung, Kyoo Park, Jong and Han, Kyoo-Seung “Electrospun PVDF nanofiber web as polymer electrolyte or separator” Electrochimica Acta 50 (2004) 339–343
- ^ (Licari, J. J., and B. L. Weigand. "Solvent-Removable Coatings for Electronic Applications." ACS Symposium Series 123 (1980): 127-37. Print.)
- ^ Chung, Y. S., S. H. Yoo, and C. K. Kim. "Enhancement of Meltdown Temperature of the Polyethylene Lithium-Ion Battery." Industrial and Engineering Chemistry Research 48.9 (2009): 4346-351. Print.
- ^ a b c Feng, J.K., Ai, X.P, Cao, Y.L. et al “A polytriphenylamine-modified separator with reversible overcharge protection for 3.6 V-class lithium-ion battery” Journal of Power Sources 189 (2009) 771–774 Cite error: The named reference "four" was defined multiple times with different content (see the help page).
- ^ Munshi, M. Z. A. Handbook of Solid State Batteries & Capacitors. Singapore: World Scientific Pub., 1995. Print.
- ^ Zhang, S.S. “A review on the separators of liquid electrolyte Li-ion batteries” Journal of Power Sources 164 (2007) 351–364
- ^ zawa, Kazunori. Lithium Ion Rechargeable Batteries: materials, Technology, and New Applications. Weinheim: WILEY-VCH, 2009. Print.
- ^ Zhang, S.S., Ervin, M.H., Xu, K., Jow, T.R.“Microporous polyacrylonitrile-methyl methacrylate membrane as a separator of rechargeable lithium battery” Electrochimica Acta 49 2004 3339–3345
- ^ Grown, S.J., Choi, J.H. , Sohn, J.Y. and et al. “Preparation of a new micro-porous poly(methyl methacrylate)-grafted polyethylene separator for high performance Li secondary battery.” Nuclear Instruments and Methods in Physics Research B 267 (2009) 3309–3313
- ^ Grown, S.J., Choi, J.H. , Sohn, J.Y. and et al. “Preparation of a new micro-porous poly(methyl methacrylate)-grafted polyethylene separator for high performance Li secondary battery.” Nuclear Instruments and Methods in Physics Research B 267 2009 3309–3313
- ^ Choi, Sung-Seen, Soo Lee, Young, Whan Joo, Chang Goo Lee, Seung, Kyoo Park, Jong and Han, Kyoo-Seung “Electrospun PVDF nanofiber web as polymer electrolyte or separator” Electrochimica Acta 50 2004 339–343
- ^ Lee, J.Y., Lee, Y.M. Bhattacharya, B. et al. “Separator grafted with siloxane by electron beam irradiation for lithium secondary batteries” Electrochimica Acta 54 2009 4312–4315
- ^ Zhang, S.S. “A review on the separators of liquid electrolyte Li-ion batteries” Journal of Power Sources 164 2007 351–364
- ^ a b Jeong, Yeon-Bok, and Dong-Won Kim. "Cycling Performances of Li/LiCoO2 Cell with Polymer-coated Separator." Electrochimica Acta 50.2-3 (2004): 323-26. Science Direct. Web. 13 Oct. 2010. Cite error: The named reference "eight" was defined multiple times with different content (see the help page).
- ^ a b Jeon, M.Y., and C.K. Kim. "Phase Behavior of Polymer/diluent/diluent Mixtures and Their Application to Control Microporous Membrane Structure." Journal of Membrane Science 300 (2007): 172-81. Print.
- ^ Nikolou, Maria. Dyer, Aubrey. Steckler, Timothy. Donoghue, Evan. Wu, Zhuangchun. Heston, Nathan. Rinzler, Andrew. Tanner, David. Reynolds, John. Dual n- and p-Type Dopable Electrochromic Devices Employing Transparent Carbon Nanotube Electrodes Chemistry of Materials 2009 21 (22), 5539-5547
- ^ Pitet, Louis M., Mark A. Amendt, and Marc A. Hillmyer. "Nanoporous Linear Polyethylene from a Block Polymer Precursor." Journal of the American Chemical Society 132.24 (2010): 8230-231. Print.
- ^ Ruxandra Vidu and Pieter Stroeve. Improvement of the Thermal Stability of Li-Ion Batteries by Polymer Coating of LiMn2O4 Industrial & Engineering Chemistry Research 2004 43 (13), 3314-3324
- ^ (Kim, J.Y. and Lim, D.Y.. “Surface-Modified Membrane as A Separator for Lithium-Ion Polymer Battery.” Energies 2010, 3(4), 866-885)
- ^ Yoo, S. H., and C. K. Kim. "Enhancement of the Meltdown Temperature of a Lithium Ion Battery Separator." Industrial and Engineering Chemistry Research 48 (2009): 99335-941. Print
- ^ Scrosati, Bruno. Applications of Electroactive Polymers. London: Chapman & Hall, 1993. Print.
- ^ Stroeve, Pieter, and Anna C. Balaz. "Macromolecular Assemblies in Polymeric Systems." ACS Symposium Series 493 (1993): 1-7. Print.
- ^ Sohna, Joon-Yong, Sung-Jin Gwon, Jae-Hak Choi, Junhwa Shina, and Young-Chang Nho. "Preparation of Polymer-coated Separators Using an Electron Beam Irradiation." Nuclear Instruments and Methods in Physics Research B (2008): 4994-5000. Print.
- ^ Chung, Y. S., S. H. Yoo, and C. K. Kim. "Enhancement of Meltdown Temperature of the Polyethylene Lithium-Ion Battery." Industrial and Engineering Chemistry Research 48.9 (2009): 4346-351. Print.
- ^ E. O. Koval’, V. V. Kolyagin, I. G. Klimov and E. A. Maier. "Investigation of the influence of technological factors on quality of basic brands of HPPE" Russian Journal of Applied Chemistry Volume 83, Number 6, 1115-1120
- ^ Jun Young Kim and Dae Young Lim. "Plasma-Modified Polyethylene Separator Membrane for Lithium-ion Polymer Battery" Next generation lithium ion batteries for electrical vehicles. 56-74.
- ^ Feng, J.K. Ai, X.P., Cao Y.L. et al. “Polytriphenylamine used as an electroactive separator material for overcharge protection of rechargeable lithium battery” Journal of Power Sources 161 (2006) 545–549
- ^ Wang, L. C., M. K. Harvey, J. C. Ng, and U. Scheunemann. "Ultra-high Molecular Weight Polyethylene UHMW-PE/ and Its." Weblogin:. Science Direct, 18 May 1998. Web. 13 Oct. 2010.
- ^ DuPont: The Miracles of Science. DuPont launches Energain separators for High-Performance Lithium Ion Batteries. http://www2.dupont.com/Automotive accessed Nov 21, 2010
- ^ DuPont: The Miracles of Science. DuPont launches Energain separators for High-Performance Lithium Ion Batteries. http://www2.dupont.com/Automotive accessed Nov 21, 2010
- ^ Kim, J. Y. Plasma-modified polyethylene membranes as a separator for lithium-ion polymer battery. Electrochimica Acta, 2009, 54(14), 3714-3719
- ^ Cai, Z. Possible application of novel solid polymer membrane gel separator in nickel/metal hydride battery. Journal of Materials Science, 2004, 39, 703-705
- ^ Kim, M; Han, G. Y.; Yoon, K. J.; Park, J. Y. Preparation of a trilayer separator and its application to lithium-ion batteries. Journal of Power Sources, 2010, 195(24), 8302-8305