User:LetMinnow/sandbox
Centrarchidae Draft 2[edit]
The centrarchid family is comprised of 38 species of fish[1], 34 of which are extant (currently living)[2].
There are eight genera included within Centrarchidae: Lepomis (Sunfishes), Micropterus (Black basses), Pomoxis (Crappie), Enneacanthus (Banded sunfishes), Centrarchus (Flier), Archoplites (Sacramento perch), Ambloplites (Rock basses), and Acantharchus (Mud sunfish) [1].
Many of the species within Centrarchidae can be separated into two main groups based on the two most common genera (Micropterus and Lepomis). Species in the genera Lepomis are defined by a deep or more round body shape, smaller mouths, and obtaining food through suction feeding [1][3]. Species in the genera Micropterus are defined by a more streamlined body shape, larger mouths, and consuming prey primarily by ram feeding methods[1][3].
Habitat[edit]
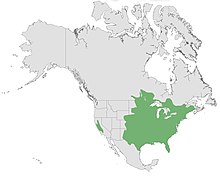
Species within the centrarchid family prefer clear, warm, and slower-moving water, and are commonly found in habitats such as lakes, ponds, streams and rivers (medium to low flow), swamps, and areas of high vegetative cover[4]. While few species in the family diverge from the aforementioned habitat list, the Sacramento perch can survive in habitats with unusually high alkalinity, salinity, and temperatures[4].
Thermal Tolerance[edit]
In freshwater systems, water temperature is determined by many abiotic factors, with air temperature being one of the most significant contributors[5]. As in other ectotherms, many physiological processes and behaviors in Centrarchidae, such as feeding and reproduction, are heavily impacted by the temperature in their environment temperature [6]. All species in the family Centrarchidae are considered warmwater adapted species[7], which are generally characterized as being larger at higher temperatures and lower latitudes and smaller in lower temperatures and higher latitudes[8]. Even though centrarchids are warmwater species, increases in temperature outside their optimal range can still have negative effects. For instance, water temperatures that are warmer than optimal have been seen to cause some centrarchids to reach reproductive maturity earlier and have higher rates of mortality after the first reproductive event[9].
The optimal temperature range of most species in the family is 28oC(82oF) to 32oC(90oF), although they can survive and reproduce in temperatures that are outside of the optimum range but within the non-lethal temperature range[3]. The lethal temperature range varies widely in the family, but some species have been seen to survive water temperatures as low as 1.7oC(35oF) or as high as 41oC(106oF)[10].
Reproduction[edit]
Centrarchids generally spawn in the spring with juveniles emerging in the late spring and early summer[11]. The transition from winter to spring conditions (i.e. melting of ice-cover, increase in day length, and increased food availability) is the main cue for centrarchids to begin preparing for reproduction[6]. All species within Centrarchidae except for those in the genus Micropterus develop breeding coloration in both the males and females (although less defined in females) during the breeding season[1].
To initiate reproduction, males will use their caudal fins to dig a deep circular depression in the substrate and create a nest[4] which they will aggressively defend from intruders[1]. Within the family, males of larger body size usually attract more mates and take better care of their offspring[12]. Males and females undergo a courtship dancing ritual before the female will deposit her eggs into the male’s nest which the male will then guard until the eggs hatch[4]. Multiple females may deposit eggs in a single nest[3]. Males not chosen during courtship may exhibit a cheater strategy where they sneak fertilizations of female’s eggs by various behavioral methods[13]. Males in Centrarchidae exhibit parental care through nest building, nest guarding, guarding of eggs and fry, and nest fanning (aerating eggs)[14].
The high levels of hybridization within Centrarchidae can be in-part attributed to the highly similar methods of courtship and reproduction among the species in the family[15]. With that said, there are some mechanisms in place to prevent hybridization, such as intricate morphology of the operculum in Lepomis, which assists in recognition of conspecific mates[1].
Range[edit]
Range Shifts[edit]
The current native range of Centrarchidae is highly restricted by the upper northern edge boundary in Canada and northern U.S.A. due to reduced foraging ability and growth in cold weather and subsequent starvation in winter months[3] [16]. The ability to adapt to cold-temperatures at the edge of the sunfish range varies widely within the family, with some species like largemouth bass (Micropterus salmoides) having no cold acclimation ability and other species like smallmouth bass (Micropterus dolomieu) and greensunfish (Lepomis cyanellus) showing signs of developing cold acclimation strategies [17]. With that said, centrarchid distributions and range in any place they are found will be monitored and restricted by cold temperatures[3].
If air temperatures continue to rise in the next 50 to 100 years as predicted[3][18], warmwater species like centrarchids will likely experience range expansions northward and see an overall increase in optimal habitat[19][5]. This range expansion can have grave consequences, though, as many centrarchids are dominant top predators which can severely alter the community structure of non-native ecosystems and drive the extinction of other native predators[20].
Invasive Range[edit]
While centrarchids are native to only North America, they can be found world-wide due to introductions within multiple continents including Europe, South America, Africa, and Asia[4]. At least 18 species of Centrarchidae are North American exports[3]. Its multi-continental spread is mostly due to the high popularity of the family (especially from the genera Micropterus) as freshwater game fish and are frequently stocked for recreational fishing all around Europe [3][4].
As highly predatory species, invasive centrarchids pose a great threat to native species in the areas they invade[21][3]. There are multiple confirmed instances of largemouth bass (Micropterus salmoides) severely altering and reducing native fish populations in Italy, South Africa, Japan, and Madagascar and even causing the local extinction of any species of the family Cyprinodontidae within the waterbodies they have invaded in Mexico[3].
_____________________________________________________________________________________________________________
First Draft: Centrarchidae[edit]
Leads Page [Additions].
The centrarchid family is comprised of 38 species of fish (Smith et al. 2015), 34 of which are extant (currently living)(Near et al. 2009).
Many of the species within Centrarchidae can be separated into two main character groups based on the two most common genera (Micropterus and Lepomis). Species in the genera Lepomis are defined by species with a deep or more round body shape, have smaller mouths, and obtain food through suction feeding (Smith et al. 2015)(Soes et al. 2010). The genera Micropterus consists of species which have more streamlined body shape, large mouths, and consume prey primarily by ram feeding methods (Smith et al. 2015)(Soes et al. 2010).
Reproduction
Centrarchids generally spawn in the spring with juveniles emerging in the late spring and early summer (Miranda 2014) with the transition from winter to spring conditions being the main cue to begin reproduction (Shuter et al. 2012). To begin reproduction, males will use their caudal fins to dig a deep circular depression in the substrate and create a nest. Male and females undergo a courtship dancing ritual before the female will deposit her eggs into the male’s nest which the male will then guard until the eggs hatch. Multiple females may deposit eggs in a single nest (Soes et al 2010). Males not chosen during courtship may exhibit a cheater strategy where they sneak fertilizations of females eggs by various behavioral methods (Gross and Charnov 1980). The high levels of hybridization within Centrarchidae can be in-part attributed to the highly similar methods of courtship and reproduction among the species in the family (Jennings and Philipp 2002). With that said, their are some mechanisms in place to prevent hybridization such as intricate morphology of the operculum in Lepomis which is found to assist in recognition of conspecific mates (Smith et al. 2015).
Impacts of Warming Temperatures
I. Thermal Tolerance
As ectotherms, many physiological processes and behaviors in centrarchids, such as feeding and reproduction, are heavily impacted by temperature (Shuter et al. 2012). In freshwater systems, water temperature is defined by many abiotic factors, air temperature being one of the most significant contributors (Lyons et al 2010). How temperatures effect any species of freshwater fish depends on its thermal strategy and optimal temperature range. All species in the family Centrarchidae are considered warmwater adapted species (Carpenter et al 1992) and have general characteristics such as being larger at higher temperatures and lower latitudes (Rypel 2014). Specifically, 28oC to 32oC was the determined range of optimal temperature of most species in the family, although they can survive and reproduce in temperatures that are outside of the optimum range but within the lethal temperature range (Soes et al. 2010). From laboratory tests measuring critical thermal maximum and critical thermal minimum of centrarchids, some species in the family could survive temperatures as low was 1.7oC or as high as 41oC (Beitingler et al. 2000). Warming temperatures near the edge of the optimal temperature range will cause desynchronization of reproduction, shifts in size-class distribution, and reduction in lifespans for freshwater fish (Jeppsen et al 2010). Warmer temperatures have been seen to cause some centrarchids to reach reproductive maturity earlier as well as have higher rates of mortality after the first reproductive event (Dembski et al 2006). Additionally, the effect that temperature shifts outside of the optimal range has on an individual can depend on its life-stage (Soes et al. 2010).
II. Range-shifts
The current native range of Centrarchidae is highly restricted by the upper northern edge boundary in Canada and northern U.S.A. due to reduced foraging ability and growth in cold weather and subsequent starvation in winter months (Soes et al. 2010) (Alofts et al. 2013). The ability to adapt to cold-temperatures at the edge of the sunfish range varies widely within the family with some species showing no ability to acclimate at all (largmouth bass) and other species showing signs of cold acclimation strategies (smallmouth bass and green sunfish)(Tschantz et al. 2002).
As air temperatures increase in the next 50 to 100 years, it will cause the increase in temperature of freshwater systems as well (Soes et al 2010). Consequently, warmwater species like centrarchids will likely experience range expansions northward and see an overall increase in optimal habitat (Comte et al 2010). The increasing temperatures will warm northern waters and reduce the severity of winter conditions making once unavailable freshwater habitat north of the native range of centrarchids available (Soes et al 2010). Due to the very limited ability for fish species to migrate, though, the increased availability of new habitat does not necessairly mean the species distribution will expand as well (Jeppsen et al. 2010). Additionally, this range expansion can have grave consequences, though, as many centrarchids are dominant top predators which can severely alter the community structure of non-native ecosystems and drive the extinction of other native predators (Near and Koppleman 2009). This can also have profoundly negative impacts for places like the Netherlands or other European countries where many centrarchids are now invasive but their distributions are still monitored by cold- temperatures (Soes et al 2010). Additionally, the homogenization of habitat due to increased temperatures will likely cause an overall decrease in the number of centrarchid species as the variation in available niche space disappears (Tisseuil et al 2012).
_____________________________________________________________________________________________________________________________________
Outline: Centrarchidae Page[edit]
Due to the highly unsatisfactory state of the entire Centrarchidae page on Wikipedia, my edits will be broken down into two phases: general page improvement and updates then information addition (new sections). The mediocrity of the Centrarchidae page can be attributed to a multitude of issues such as incorrect data, lack of sufficient information, and faulty sentence structure. My planned improvements of the existing materials include addition of citations throughout the page, modification of run-on and incomplete sentences, and finally small additions of pertinent information to the lead section and habitat section.
In addition to my general improvements, I will be formulating and adding two new sections to the page. The first is a section over reproduction in which I will briefly outline the major reproductive strategies within the centrarchid family. The second section, Impacts of Climate Change, will be a much larger second addition. This section will be divided into two subsections will focus: thermal tolerance and range shifts.
In the thermal tolerance subsection I will discuss the optimal temperature regimes of centrarchids and how current warming trends are affecting those tolerances. In the range shifts section I will discuss the current state of knowledge of the impacts of warming on the distribution of Centrarchidae and how those shifts are predicted to effect species dispersal and overall abundance.
Current organization of topics.
1. Fossil Record
2. Habitat
3. Classification
My Proposed Adjustments.
1. Fossil Record
2. Habitat
3. Reproduction
4. Climate Change
i. Thermal tolerance
ii. Range sifts
5. Classification
 | This is a user sandbox of LetMinnow. You can use it for testing or practicing edits. This is not the sandbox where you should draft your assigned article for a dashboard.wikiedu.org course. To find the right sandbox for your assignment, visit your Dashboard course page and follow the Sandbox Draft link for your assigned article in the My Articles section. |
- ^ a b c d e f g Smith, Andrew J.; Nelson-Maney, Nathan; Parsons, Kevin J.; Cooper, W. James; Albertson, R. Craig (2015-09-01). "Body Shape Evolution in Sunfishes: Divergent Paths to Accelerated Rates of Speciation in the Centrarchidae". Evolutionary Biology. 42 (3): 283–295. doi:10.1007/s11692-015-9322-y. ISSN 0071-3260. S2CID 17580461.
- ^ Near, Thomas J.; Kassler, Todd W.; Koppelman, Jeffrey B.; Dillman, Casey B.; Philipp, David P.; Orti, G. (2003-07-01). "Speciation in north american black basses, micropterus (actinopterygii: centrarchidae)". Evolution. 57 (7): 1610–1621. doi:10.1554/02-295. ISSN 0014-3820. PMID 12940365. S2CID 198155858.
- ^ a b c d e f g h i j k Soes, Menno; Cooke, Steven; van Kleef, H.H.; Broeckx, P.B.; Veenvliet, P. (March 21, 2010). "A risk analysis of sunfishes (Centrarchidae) and pygmy sunfishes (Elassomatidae) in the Netherlands". Bureau of Waardenburg Bv. Report nr 11-042: 1–110 – via ResearchGate.
{{cite journal}}: CS1 maint: date and year (link) - ^ a b c d e f Berra, Tim (2007). Freshwater Fish Distribution. Chicago and London: The University of Chicago Press. pp. 390–400. ISBN 978-0-226-04442-2.
- ^ a b Lyons, J.; Stewart, J. S.; Mitro, M. (2010-11-01). "Predicted effects of climate warming on the distribution of 50 stream fishes in Wisconsin, U.S.A." Journal of Fish Biology. 77 (8): 1867–1898. doi:10.1111/j.1095-8649.2010.02763.x. ISSN 1095-8649. PMID 21078096.
- ^ a b Shuter, B. J.; Finstad, A. G.; Helland, I. P.; Zweimüller, I.; Hölker, F. (2012-10-01). "The role of winter phenology in shaping the ecology of freshwater fish and their sensitivities to climate change". Aquatic Sciences. 74 (4): 637–657. doi:10.1007/s00027-012-0274-3. ISSN 1015-1621. S2CID 18297554.
- ^ Carpenter, Stephen R.; Fisher, Stuart G.; Grimm, Nancy B.; Kitchell, James F. (1992-11-01). "Global Change and Freshwater Ecosystems". Annual Review of Ecology and Systematics. 23 (1): 119–139. doi:10.1146/annurev.ecolsys.23.1.119. ISSN 0066-4162.
- ^ Rypel, Andrew L. (2014-01-01). "The Cold-Water Connection: Bergmann's Rule in North American Freshwater Fishes". The American Naturalist. 183 (1): 147–156. doi:10.1086/674094. ISSN 0003-0147. PMID 24334744. S2CID 22642325.
- ^ Dembski, S.; Masson, G.; Monnier, D.; Wagner, P.; Pihan, J. C. (2006-08-01). "Consequences of elevated temperatures on life-history traits of an introduced fish, pumpkinseed Lepomis gibbosus". Journal of Fish Biology. 69 (2): 331–346. doi:10.1111/j.1095-8649.2006.01087.x. ISSN 1095-8649.
- ^ Beitinger, Thomas L.; Bennett, Wayne A.; McCauley, Robert W. (2000-07-01). "Temperature Tolerances of North American Freshwater Fishes Exposed to Dynamic Changes in Temperature". Environmental Biology of Fishes. 58 (3): 237–275. doi:10.1023/A:1007676325825. ISSN 0378-1909. S2CID 35400804.
- ^ Miranda, L.E. (Steve) (Winter 2014). "Fish in Winter – Changes in Latitudes, Changes in Attitudes" (PDF). Lake Line (a publication of the North American Lake Management Society). Vol. 34, no. 4. pp. 28–31.
- ^ Danylchuk, Andy J.; Fox, Michael G. (1996-10-01). "Size- and age-related variation in the seasonal timing of nesting activity, nest characteristics, and female choice of parental male pumpkinseed sunfish (Lepomis gibbosus)". Canadian Journal of Zoology. 74 (10): 1834–1840. doi:10.1139/z96-206. ISSN 0008-4301.
- ^ Gross, Mart R.; Charnov, Eric L. (1980-11-01). "Alternative male life histories in bluegill sunfish". Proceedings of the National Academy of Sciences. 77 (11): 6937–6940. doi:10.1073/pnas.77.11.6937. PMC 350407. PMID 16592922.
- ^ Blumer, Lawrence S. (1982-05-01). "A bibliography and categorization of bony fishes exhibiting parental care". Zoological Journal of the Linnean Society. 75 (1): 1–22. doi:10.1111/j.1096-3642.1982.tb01939.x. hdl:2027.42/71841. ISSN 0024-4082.
- ^ Jennings, Martin J.; Philipp, David P.; Montgomery, W. L. (2002-12-01). "Alternative Mating Tactics in Sunfishes (Centrarchidae): A Mechanism for Hybridization?". Copeia. 2002 (4): 1102–1105. doi:10.1643/0045-8511(2002)002[1102:amtisc]2.0.co;2. ISSN 0045-8511. S2CID 85691602.
- ^ Alofs, Karen M.; Jackson, Donald A.; Lester, Nigel P. (2014-02-01). "Ontario freshwater fishes demonstrate differing range-boundary shifts in a warming climate". Diversity and Distributions. 20 (2): 123–136. doi:10.1111/ddi.12130. ISSN 1472-4642. S2CID 85651008.
- ^ Tschantz, Deidra R.; Crockett, Elizabeth L.; Niewiarowski, Peter H.; Londraville, Richard L. (2002-11-01). "Cold Acclimation Strategy Is Highly Variable among the Sunfishes (Centrarchidae)". Physiological and Biochemical Zoology. 75 (6): 544–556. doi:10.1086/344492. ISSN 1522-2152. PMID 12601611. S2CID 36858254.
- ^ Piggott, Jeremy J.; Townsend, Colin R.; Matthaei, Christoph D. (2015-05-01). "Climate warming and agricultural stressors interact to determine stream macroinvertebrate community dynamics". Global Change Biology. 21 (5): 1887–1906. doi:10.1111/gcb.12861. ISSN 1365-2486. PMID 25581853. S2CID 25594059.
- ^ COMTE, LISE; BUISSON, LAËTITIA; DAUFRESNE, MARTIN; GRENOUILLET, GAËL (2013-04-01). "Climate-induced changes in the distribution of freshwater fish: observed and predicted trends". Freshwater Biology. 58 (4): 625–639. doi:10.1111/fwb.12081. ISSN 1365-2427.
- ^ Near, T. J.; Koppelman, J. B. Centrarchid Fishes. Wiley-Blackwell. pp. 1–38. doi:10.1002/9781444316032.ch1.
- ^ Sterud, Erik (2006). "Pumpkinseed Lepomis gibbosus (Centrarchidae) and associated parasites introduced to Norway". Aquatic Invasions. 1 (4): 278–280. doi:10.3391/ai.2006.1.4.10.
