User:Lena08041993/PaliperidoneExample
This article may be unbalanced toward certain viewpoints. (September 2014) |
 | |
| Clinical data | |
|---|---|
| Trade names | Invega |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a607005 |
| License data |
|
| Pregnancy category |
|
| Routes of administration | Oral (OROS tablets), IM depot injection |
| ATC code | |
| Legal status | |
| Legal status | |
| Pharmacokinetic data | |
| Bioavailability | 28% (oral) |
| Elimination half-life | 23 hours (oral) |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| ChEMBL | |
| Chemical and physical data | |
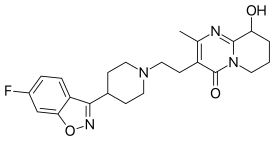
| Formula | C23H27FN4O3 |
| Molar mass | 426.484 g/mol g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| | |
Paliperidone (trade name Invega), also known as 9-hydroxyrisperidone, is a dopamine antagonist and 5-HT2A antagonist of the atypical antipsychotic class of medications. It is marketed by Janssen Pharmaceutica. Invega is an extended release formulation of paliperidone that uses the OROS extended release system to allow for once-daily dosing.
Paliperidone palmitate (trade name Invega Sustenna, named Xeplion in Europe and other countries) is a long-acting injectable formulation of paliperidone palmitoyl ester indicated for once-every 28 days injection after an initial titration period. Paliperidone is used to treat mania and at lower doses as maintenance for bipolar disorder. It is also indicated in the US by the FDA for schizophrenia and schizoaffective disorder.
On May 18, 2015, a new formulation of paliperidone palmitate was approved by the FDA under the brand name Invega Trinza.[1] A similar 3 -monthly injection of prolonged release suspension was approved in 2016 by the European Medicines Agency originally under the brand name Paliperidone Janssen, later renamed to Trevicta.[2]
Medical use[edit]
It is used for the treatment of schizophrenia and schizoaffective disorder. In a 2013 study in a comparison of 15 antipsychotic drugs in effectivity in treating schizophrenic symptoms, paliperidone was ranked fifth, demonstrating medium-strong effectivity. Less effective than clozapine, slightly less effective than olanzapine and risperidone, and slightly more effective than haloperidol, quetiapine, and aripiprazole.[3]
| Summary | ||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| In short-term studies, paliperidone palmitate - the longer-acting injection - is an antipsychotic drug with a similar adverse effect profile to related compounds such as oral risperidone. No difference was found in the [high] incidence of reported adverse sexual outcomes and paliperidone palmitate is associated with substantial increases in serum prolactin. When flexibly dosed with an average dose of approximately 70-110 mg every four weeks, paliperidone palmitate appears comparable in efficacy and tolerability to risperidone.[4] | ||||||||||||||||||||||||||||||||||||||||||||||||
| ||||||||||||||||||||||||||||||||||||||||||||||||
Adverse effects[edit]
- Very Common (>10% incidence)
- Headache
- Tachycardia
- Somnolence (causes less sedation than most atypical antipsychotics[9])
- Insomnia
- Hyperprolactinaemia (seems to cause comparable prolactin elevation to its parent drug, risperidone[9])
- Common (1–10% incidence)
- Cough
- Extrapyramidal side effects (EPSE; e.g. dystonia, akathisia, muscle rigidity, parkinsonism. It appears to produce similar EPSE to risperidone, asenapine and ziprasidone and more EPSE than olanzapine, clozapine, aripiprazole, quetiapine, amisulpride and sertindole[9])
- Orthostatic hypotension
- Weight gain (tends to produce a moderate degree of weight gain, possibly related to its potent blockade of the 5-HT2C receptor)
- QT interval prolongation (tends to produce less QT interval prolongation than most other atypical antipsychotics and approximately as much QT interval prolongation as aripiprazole and lurasidone[9])
- Nasopharyngitis
- Anxiety
- Indigestion
- Constipation
Deaths[edit]
In April 2014, it was reported that 21 Japanese people who had received shots of the long-acting injectable paliperidone to date had died.[10][11][12][13][14][15][16]
Pharmacology[edit]
Paliperidone is the primary active metabolite of the older antipsychotic risperidone.[17] While its specific mechanism of action is unknown, it is believed paliperidone and risperidone act via similar, if not identical, pathways.
Paliperidone has antagonist effect at α1 and α2 adrenergic receptors and at H1 histamine receptors.[18] It does not bind to muscarinic acetylcholine receptors. In addition, it blocks dopamine and serotonin receptors.
Paliperidone has less affinity for D4 receptors than risperidone.[19][20]
Food increases the absorption of Invega type ER OROS prolonged-release tablet. Food increased exposure of Paliperidone by up to 50-60%, however, half-life was not significantly affected. The effect was probably due to a delay in the transit of the ER OROS formulation in the upper part of the GI channel, resulting in increased absorption (uptake).[21]
The half life is 24 hours.[21]
History[edit]
Paliperidone (as Invega) was approved by the Food and Drug Administration (FDA) for the treatment of schizophrenia in 2006. Paliperidone was approved by the FDA for the treatment of schizoaffective disorder in 2009. The long-acting injectable form of paliperidone, marketed as Invega Sustenna in U.S. and Xeplion in Europe, was approved by the FDA on July 31, 2009.
It was initially approved in Europe in 2007 for schizophrenia, the extended release form and use for schizoaffective disorder were approved in Europe in 2010, and extension to use in adolescents older than 15 years old was approved in 2014.[22]
References[edit]
- ^ "Invega Trinza™ (paliperidone palmitate) NDA approval letter" (PDF). U.S. Food and Drug Administration. Retrieved 10 December 2015.
- ^ "Trevicta (previously Paliperidone Janssen) - summary of the European public assessment report (EPAR) for Trevicta".
- ^ Leucht, Stefan; Cipriani, Andrea; Spineli, Loukia; Mavridis, Dimitris; Örey, Deniz; Richter, Franziska; Samara, Myrto; Barbui, Corrado; Engel, Rolf R; Geddes, John R; Kissling, Werner; Stapf, Marko Paul; Lässig, Bettina; Salanti, Georgia; Davis, John M (September 2013). "Comparative efficacy and tolerability of 15 antipsychotic drugs in schizophrenia: a multiple-treatments meta-analysis". The Lancet. 382 (9896): 951–962. doi:10.1016/S0140-6736(13)60733-3. PMID 23810019. S2CID 32085212.
- ^ a b Nussbaum, A; Stroup, T (2012). "Paliperidone palmitate for schizophrenia". Cochrane Database of Systematic Reviews. 6 (6): CD008296.pub2. doi:10.1002/14651858.CD008296.pub2. PMID 22696377.
- ^ Truven Health Analytics, Inc. DrugPoint® System (Internet) [cited 2013 Sep 30]. Greenwood Village, CO: Thomsen Healthcare; 2013.
- ^ INVEGA® PRODUCT INFORMATION [Internet]. Janssen Pharmaceuticals; 2013 [cited 2013 Sep 30]. Available from: https://www.ebs.tga.gov.au/ebs/picmi/picmirepository.nsf/pdf?OpenAgent&id=CP-2013-PI-01421-1
- ^ Joint Formulary Committee. British National Formulary (BNF) 65. Pharmaceutical Pr; 2013.
- ^ paliperidone (Rx) - Invega, Invega Sustenna [Internet]. Medscape Reference. [cited 2013 Sep 30]. Available from: http://reference.medscape.com/drug/invega-sustenna-paliperidone-342992#4
- ^ a b c d Leucht, Stefan; Cipriani, Andrea; Spineli, Loukia; Mavridis, Dimitris; Örey, Deniz; Richter, Franziska; Samara, Myrto; Barbui, Corrado; Engel, Rolf R; Geddes, John R; Kissling, Werner; Stapf, Marko Paul; Lässig, Bettina; Salanti, Georgia; Davis, John M (2013). "Comparative efficacy and tolerability of 15 antipsychotic drugs in schizophrenia: a multiple-treatments meta-analysis". Lancet. 382 (9896): 951–62. doi:10.1016/S0140-6736(13)60733-3. PMID 23810019. S2CID 32085212.
- ^ 21 users of schizophrenia drug dead | The Japan Times
- ^ Schizophrénie: controverse autour d'un médicament au Japon | Médecine
- ^ 20 minutes - Un médicament anti-schizophrénie tue - Monde
- ^ Deaths reported after Xeplion injections - Life & Style - NZ Herald News
- ^ 17 deaths reported after schizophrenia drug injections | Japan Today: Japan News and Discussion
- ^ 21 Dead in Japan From New Johnson & Johnson Antipsychotic | Mad In America
- ^ Schizophrenia drug blamed for 17 deaths | Sky News Australia
- ^ "The DrugBank database".
- ^ "Prescribing Reference: New Product Releases - INVEGA". Retrieved 2008-05-25.
- ^ http://pdsp.med.unc.edu/pdsp.php?knowID=&kiKey=&receptorDD=&receptor=&speciesDD=&species=&sourcesDD=&source=&hotLigandDD=&hotLigand=&testDDRadio=testDDRadio&testLigandDD=2327&testLigand=&referenceDD=&reference=&KiGreater=&KiLess=&kiAllRadio=all&doQuery=Submit+Query[full citation needed]
- ^ http://pdsp.med.unc.edu/pdsp.php?knowID=&kiKey=&receptorDD=&receptor=&speciesDD=&species=&sourcesDD=&source=&hotLigandDD=&hotLigand=&testDDRadio=testDDRadio&testLigandDD=2328&testLigand=&referenceDD=&reference=&KiGreater=&KiLess=&kiAllRadio=all&doQuery=Submit+Query[full citation needed]
- ^ a b "Paliperidone extended release: Scientific Discussion" (PDF). EMA. 16 July 2007. Linked from EMA main page on drug, in "Assessment History" tab.
- ^ "Procedural steps taken and scientific information after the authorisation" (PDF). EMA. 16 July 2015. Linked from EMA main page on drug, in "Assessment History" tab.
