User:Keppra28/sandbox
| |||
| Clinical data | |||
|---|---|---|---|
| Pronunciation | /lɛv[invalid input: 'ɨ']tɪˈræs[invalid input: 'ɨ']tæm/ | ||
| AHFS/Drugs.com | Monograph | ||
| MedlinePlus | a699059 | ||
| License data |
| ||
| Pregnancy category |
| ||
| Routes of administration | Oral, intravenous | ||
| ATC code | |||
| Legal status | |||
| Legal status | |||
| Pharmacokinetic data | |||
| Bioavailability | ~100% | ||
| Protein binding | <10% | ||
| Metabolism | Enzymatic hydrolysis of acetamide group | ||
| Elimination half-life | 6 - 8 hr | ||
| Excretion | Urinary | ||
| Identifiers | |||
| |||
| CAS Number | |||
| PubChem CID | |||
| IUPHAR/BPS | |||
| DrugBank | |||
| ChemSpider | |||
| UNII | |||
| KEGG | |||
| ChEBI | |||
| ChEMBL | |||
| Chemical and physical data | |||
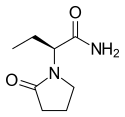
| Formula | C8H14N2O2 | ||
| Molar mass | 170.209 g/mol g·mol−1 | ||
| 3D model (JSmol) | |||
| |||
| |||
| | |||
Levetiracetam is an anticonvulsant medication used to treat epilepsy.[1] It is the S-enantiomer of etiracetam, structurally similar to the prototypical nootropic drug piracetam.
Levetiracetam is marketed under the trade name Keppra. Keppra is manufactured by UCB Pharmaceuticals Inc. Since November 2008, the drug is available as a generic brand in the United States and the United Kingdom.
Medical uses[edit]
Levetiracetam has been approved in the European Union as a monotherapy treatment for epilepsy in the case of partial seizures, or as an adjunctive therapy for partial, myoclonic and tonic-clonic seizures.[2] It is also used in veterinary medicine for similar purposes.
Levetiracetam has potential benefits for other psychiatric and neurologic conditions such as Tourette syndrome, autism, and anxiety disorder,[3] as well as Alzheimer's disease.[4] However, due to possible adverse effects, its benefit-risk ratio in these conditions is not well understood.[3]
Along with other anticonvulsants like gabapentin, it is also sometimes used to treat neuropathic pain. It has not been found to be useful for essential tremors.[5]
Mechanism of action[edit]
The exact mechanism by which levetiracetam acts to treat epilepsy is unknown. However, the drug binds to a synaptic vesicle glycoprotein, SV2A,[6] and inhibits presynaptic calcium channels [7] reducing neurotransmitter release and acting as a neuromodulator. This is believed to impede impulse conduction across synapses.[8]
Pharmacodynamics[edit]
Pharmacokinetics[edit]
Absorption[edit]
Distribution[edit]
Metabolism[edit]
Excretion[edit]
Adverse effects[edit]
The most common adverse effects of levetiracetam treatment include CNS effects such as somnolence, infection, asthenia, headache, dizziness, and ataxia. These adverse effects are most pronounced in the first month of therapy. About 4% of patients dropped out of pre-approval clinical trials due to these side effects.
About 13% of people taking levetiracetam experience adverse neuropsychiatric symptoms, which are usually mild. These include including agitation, hostility, apathy, anxiety, emotional lability, and depression. Serious psychiatric adverse side effects that are reversed by drug discontinuation occur in about 1%. These include hallucinations, suicidal ideations, or psychosis. These occurred mostly within the first month of therapy, but they could develop at any time during treatment.[9]
A study published in 2005 suggests that the addition of pyridoxine (vitamin B6) may curtail some of the psychiatric symptoms.[10]
Although rare, Stevens–Johnson syndrome (SJS) and toxic epidermal necrolysis (TEN), have been reported in patients treated with levetiracetam. Recommendations are to discontinue leviteracetam upon signs of unexplained rash. If signs or symptoms suggest SJS/TEN, use of this drug should not be resumed and alternative therapy should be considered. The incidence of SJS following exposure to anti-epileptics such as Levetiracetam is about 1 in 3,000[11]
Warnings[edit]
Levetiracetam, along with other anti-epileptic drugs, can increase the risk of suicide behavior or ideation. Patients taking levetiracetam should be monitored closely for signs of worsening depression, suicidal thoughts or tendencies, or any altered emotional or behavioral states. [10]
Levetiracetam should not be used in patients who have previously shown hypersensitivity to levetiracetam or any of the inactive ingredients in the tablet or oral solution. Such hypersensitivity reactions include, but are not limited to, unexplained rash with redness or blistered skin, difficulty breathing, and tightness in the chest or airways. [11]
Drug interactions[edit]
No significant pharmacokinetic interactions were observed between levetiracetam or its major metabolite and concomitant medications.[12] The pharmacokinetic profile of Keppra is not influenced by phenytoin, phenobarbital, primidone, carbamazepine, valproic acid, lamotrigine, gabapentin, digoxin, oral contraceptives ethinylestradiol, and warfarin.[13]
Special populations[edit]
Pregnancy[edit]
Levetiracetam is a Pregnancy Category C Drug. Studies in female pregnant rats have shown minor fetal skeletal abnormalities when given maximum recommended human doses of Keppra orally throughout pregnancy and lactation.[14]
A study in the Journal Neurology retrospectively looked at 671 human pregnancies with known maternal exposure to levetiracetam and found that the rate of Major congenital malformations (MCM) was not significantly higher when levetiracetam was used as a monotherapy. However, the majority of the patients were also exposed to other anti-epileptic drugs as a combination therapy and found increases in Major Congential Malformations when combined with valproate and carbamazepine. The paper concluded that the data suggests levetiracetam monotherapy to be a suitable regimen if anti-epileptic medication is needed during pregnancy.[15]
Elderly[edit]
Studies were conducted to look for increased adverse effects in the elderly population as compared to younger patients. One such study published in Epilepsy Research showed no significant increase in incidence of adverse symptoms experience by young and elderly patients with CNS disorders.[16]
Children[edit]
No established safety and efficacy for use in patients under 4 years of age. Animal studies in juvenile rats and dogs did not indicate a potential for age-specific toxicity.[17]
Measurement in bodily fluids[edit]
Assay of levetiracetam[edit]
There are only a few papers published reporting therapeutic drug monitoring methods of levetiracetam. Three of them employed HPLC with UV-detection,[18][19][20] and two methods were using GC with NPD-detection.[19][21] Microemulsion electrokinetic chromatography with UV-detection was utilized in one method.[22] Two methods facilitating chiral separation of the S- and R- enantiomer of levetiracetam, one utilizing GC–MS and the other HPLC–UV, were published.[23][24] These methods were designed to investigate in dogs the pharmacokinetic and pharmacodynamic properties of the two enantiomers separately. For routine therapeutic drug monitoring in men, these methods were not appropriate. In all but one of the methods,[20] sample preparation with SPE or liquid–liquid extraction is necessary. Pucci et al.[20] evaluated the feasibility of protein precipitation as the only sample preparation step in comparison to SPE. They concluded, that protein precipitation is a suitable and fast sample preparation for measuring routine patient samples. Mecarelli et al.[25] studied the concentration of levetiracetam in both serum and saliva of patients with epilepsy.
Various HPLC,[26][27][28][29][30][31][32] and LC-MS,[33][34][35] methods have been reported for the determination of levetiracetam in pure and pharmaceutical dosage forms.
Available forms[edit]
Avaliable as intravenous and oral formulations.
In 2015 Aprecia’s 3d-printed form of the drug was approved by the FDA.[36]
See also[edit]
References[edit]
- ^ Abou-Khalil B (June 2008). "Levetiracetam in the treatment of epilepsy". Neuropsychiatr Dis Treat. 4 (3): 507–23. doi:10.2147/NDT.S2937. PMC 2526377. PMID 18830435.
{{cite journal}}: CS1 maint: unflagged free DOI (link) - ^ BNF 59. BMA & RPSGB. 2010.
- ^ a b Farooq MU, Bhatt A, Majid A, Gupta R, Khasnis A, Kassab MY (2009). "Levetiracetam for managing neurologic and psychiatric disorders". Am J Health Syst Pharm. 66 (6): 541–61. doi:10.2146/ajhp070607. PMID 19265183.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Sanchez, Pascal; Zhu, Verret; Vossel, Orr; Cirrito, Devidze; Ho, Yu; Palop, Mucke (August 6, 2012). "Levetiracetam suppresses neuronal network dysfunction and reverses synaptic and cognitive deficits in an Alzheimer's disease model". PNAS. 109 (42): E2895–903. doi:10.1073/pnas.1121081109. PMC 3479491. PMID 22869752.
- ^ Zesiewicz, TA (Nov 8, 2011). "Evidence-based guideline update: treatment of essential tremor: report of the Quality Standards subcommittee of the American Academy of Neurology". Neurology. 77 (19): 1752–5. doi:10.1212/WNL.0b013e318236f0fd. PMC 3208950. PMID 22013182.
{{cite journal}}: Unknown parameter|coauthors=ignored (|author=suggested) (help) - ^ Lynch BA, Lambeng N, Nocka K, et al. (June 2004). "The synaptic vesicle protein SV2A is the binding site for the antiepileptic drug levetiracetam". Proc Natl Acad Sci USA. 101 (26): 9861–6. doi:10.1073/pnas.0308208101. PMC 470764. PMID 15210974.
- ^ Vogl C, Mochida S, Wolff C, et al. (August 2012). "The Synaptic Vesicle Glycoprotein 2A Ligand Levetiracetam Inhibits Presynaptic Ca2+ Channels through an Intracellular Pathway". Mol Pharmacol. 82 (2): 199–208. doi:10.1124/mol.111.076687. PMID 22554805. S2CID 8333373.
- ^ Rogawski, MA (June 2006). "Diverse mechanisms of antiepileptic drugs in the development pipeline". Epilepsy Research. 69 (3): 273–94. doi:10.1016/j.eplepsyres.2006.02.004. PMC 1562526. PMID 16621450.
- ^ Gambardella A, Labate A, Colosimo E, Ambrosio R, Quattrone A (February 2008). "Monotherapy for partial epilepsy: focus on levetiracetam". Neuropsychiatr Dis Treat. 4 (1): 33–8. doi:10.2147/NDT.S1655. PMC 2515905. PMID 18728811.
{{cite journal}}: CS1 maint: multiple names: authors list (link) CS1 maint: unflagged free DOI (link) - ^ "Clinical Epilepsy: Pediatrics". Epilepsia. 46 (s8): 142–67. 2005. doi:10.1111/j.1528-1167.2005.460801_16.x. S2CID 221731585.
- ^ Griebel ML. Acute management of hypersensitivity reactions and seizures. Epilepsia. 1998;39(7):S17–S21
- ^ Browne TR, Szabo GK, Leppik IE, et al. Absence of pharmacokinetic drug interaction of levetiracetam with phenytoin in patients with epilepsy determined by new technique. J Clin Pharmacol. 2000;40:590–5.
- ^ Gidal BE, Baltès E, Otoul C, et al. Effect of levetiracetam on the pharmacokinetics of adjunctive antiepileptic drugs: a pooled analysis of data from randomized clinical trials. Epilepsy Res.2005;64:1–11.
- ^ "HIGHLIGHTS OF PRESCRIBING INFORMATION" (PDF). UCB, Inc. Retrieved 29 May 2014.
- ^ Mawhinney E, Craig J, Morrow J,et al. Levetiracetam in pregnancy results from the UK and Ireland epilepsy and pregnancy registers. Neurology 2013;80:400–405.
- ^ Cramer, J.A., Leppik, I.E., De Rue, K., Edrich, P., Krämer, G. Tolerability of levetiracetam in elderly patients with CNS disorders (2003) Epilepsy Research, 56 (2-3), pp. 135-145.
- ^ "DailyMed - KEPPRA- levetiracetam tablet". dailymed.nlm.nih.gov. Retrieved 2015-11-04.
- ^ N. Ratnaraj, H.C. Doheny, P.N. Patsalos, Ther. Drug Monit. 18 (1996) 154.
- ^ a b T.A. Vermeij, P.M. Edelbroek, J. Chromatogr. B Biomed. Appl. 662 (1994) 134.
- ^ a b c V. Pucci, F. Bugamelli, R. Mandrioli, A. Ferranti, E. Kenndler, M.A. Raggi, Biomed. Chromatogr. 18 (2004) 37.
- ^ R. Coupez, R. Straetemans, G. Sehgal, A. Stockis, Z.S. Lu, J. Clin. Pharmacol. 43 (2003) 1370.
- ^ M. Ivanova, A. Piunti, E. Marziali, N. Komarova, M.A. Raggi, E. Kenndler, Electrophoresis 24 (2003) 992.
- ^ N. Isoherranen, M. Roeder, S. Soback, B. Yagen, V. Schurig, M. Bialer, J. Chromatogr. B Biomed. Sci. Appl. 745 (2000) 325.
- ^ N. Isoherranen, B. Yagen, S. Soback, M. Roeder, V. Schurig, M. Bialer, Epilepsia 42 (2001) 825.
- ^ O. Mecarelli, P. Li Voti, S. Pro, F.S. Romolo, M. Rotolo, P. Pulitano, N. Accornero, N. Vanacore, Saliva and serum levetiracetam concentrations in patients with epilepsy. Therapeutic Drug Monitoring (2007), 29(3), 313-318.
- ^ Prafulla Kumar Sahu*, Dillip Kumar Sahoo, M.M.Annapurna, M.E.Bhanoji Rao, Development and validation of an RP-HPLC method for determination of Levetiracetam in Bulk and Pharmaceutical Dosage Forms, Analytical Chemistry: An Indian Journal, 2009, 8(1).
- ^ C. Manuela, M. Susan, A. Fiorenzo, R. Roberto and B. Agostino, J chromatogr B., 2008, 873(1), 129.
- ^ N. Appala Raja, J. Venkateswara Rao, K. Vanitha Prakash, K. Mukkanti and K. Srinivasu, E Journal of Chemistry, 2008, 5(S2), 1098.
- ^ J. Valarmathy, L. Samueljoshua, G. Rathinavel, C. Selvin Thanija and T. Sivakumar,Research J Pharm and Tech., 2008, 1(3), 395.
- ^ J. Marten Lobenhoffer and S.M. Bode Boger, J Chromatogr B., 2005, 815, 197.
- ^ N. Ratnaraj, C. Doheny Helen and N. Patsalos Philip, Ther Drug Monit, 1996, 18(2), 154.
- ^ A.C. Vermeij and P.M. Edelbroek, J Chromatogr B., 1994, 662,134.
- ^ M. Kamal Matar, J Pharm Biomed Anal., 2008, 48(3), 822.
- ^ G. Saravanan, G. Jhothy, Y. Suresh, A. Annerao, M. Ramakrishna, M. Yogeshwar Reddy and B. Ravibabu, Chromatographia, 2008, 67, 173.
- ^ Tiedong Guo, M. Lisa Oswald, M. Damodara Rao and J. Steven Soldin, Clinica Chimica Acta, 2007, 375, 115.
- ^ "FDA approves the first 3D-printed drug product | KurzweilAI". www.kurzweilai.net. October 13, 2015. Retrieved 2015-10-14.
External links[edit]
- PubMed Health A division of the National Library of Medicine at the National Institutes of Health.
- Keppra (levetiracetam) Final Printed Label April 2009. Center for Drug Evaluation and Research, U.S. Food and Drug Administration. Accessed 29 July 2011.
- Keppra UCB (manufacturer's website)
- NIH MedLine drug information
Category:Racetams Category:Anticonvulsants Category:Pyrrolidones Category:Enantiopure drugs Category:Butyramides Category:Belgian inventions