User:Jcap17/sandbox
The Sonogashira cross-coupling reaction is used in organic synthesis to form carbon-carbon bonds. It makes use of a palladium catalyst to form a carbon-carbon bond between a terminal alkyne and a sp2organic halide or psuedohalide.[1]

The Sonogashira cross-coupling reaction has been employed in a wide variety of areas, due to its usefulness in the formation of carbon-carbon bonds. The reaction can be carried out under mild conditions, such as at room temperature, in aqueous media, and with a mild base, which has allowed for the use of the Sonogashira cross-coupling reaction in the synthesis of complex molecules. Its applications include pharmaceuticals, natural products, organic materials, and nanomaterials.[1] Specific examples include its use in the synthesis of Tazarotene, which is a treatment for psoriasis and acne, and in the preparation of SIB-1508Y, also known as Altinicline, which is a potential treatment for Parkinson’s disease, Alzheimer’s disease, Tourette’s syndrome, Schizophrenia, and attention deficit hyperactivity disorder (ADHD).[2]
History[edit]
The Sonogashira cross-coupling reaction was first reported by Kenkichi Sonogashira, Yasuo Tohda, and Nobue Hagihara in their 1975 publication.[3] It is an extension to the Cassar and Dieck and Heck reactions, which afford the same reaction products, but use harsh reaction conditions, such as high temperature, to do so. Both of these reactions make use of a palladium catalyst to carry out the coupling, while Sonogashira uses both palladium and copper cataylysts simultaneously. This results in the increased reactivity of the reagents and the ability of the reaction to be carried out at room temperature, making the Sonogashira cross-coupling reaction a highly useful reaction.[4] Its true usefulness can be seen in the amount of research still being done on it. A search for the term "Sonogashira" in Scifinder provides over 1500 references for journal publications between 2007 and 2010.[4] It has become so well known that often, all reactions that use a palladium(0) catalyst to couple a sp2 and even sp3 halide or triflate with a terminal alkyne, regardless of whether or not a copper co-catalyst is used, are termed "Sonogashira reactions," despite the fact that these reactions are not carried out under true Sonogashira reaction conditions.[4]
Mechanism[edit]
The reaction mechanism is not clearly understood, but the textbook mechanism revolves around a classical palladium cycle and a copper cycle that is less well known.[5]

The palladium cycle[edit]
- The active palladium catalyst is the 14 electron compound Pd0L2, complex A, which reacts with the aryl or vinyl halide in an oxidative addition to produce a PdII intermediate, complex B. This step is believed to be the rate-limiting step of the reaction.
- Complex B reacts in a transmetallation with the copper acetylide, complex F, which is produced in the copper cycle, to give complex C, expelling the copper halide, complex G.
- Both organic ligands are trans oriented and convert to cis in a trans-cis isomerization to produce complex D.
- In the final step, complex D undergoes reductive elimination to produce the alkyne, with regeneration of the palladium catalyst.
The copper cycle[edit]
- It is suggested that the presence of base results in the formation of a pi-alkyne complex, complex E, which makes the terminal proton on the alkyne more acidic, leading to the formation of the copper acetylide, compound F.
- Compound F continues to react with the palladium intermediate B, with regeneration of the copper halide, G.
Mechanistic studies suggest that these catalytic cycles represent the preferred reaction pathway, however there is debate about the exact identity of some intermediates, which may depend upon reaction conditions. For example, it has been shown that monoligated Pd0(PR3) complexes (B) can be formed when dealing with bulky phosphanes and have been suggested as possible catalytic species in coupling reactions.[6] In contrast, some results point to the formation of anionic palladium species, which would be the real catalysts instead of the coordinatively unsaturated Pd0L2. Generally seen in the presence of anions and halides, it is known that Pd0(PPh3)2 does not exist in solution when generated in the presence of halide anions because they coordinate the Pd0 center to form anionic species of the type [L2Pd0Cl]- which can participate in cross-coupling reactions.[7]
Catalysts[edit]
Typically, two catalysts are needed for this reaction: a zerovalent palladium complex and a halide salt of copper(I). Examples of such palladium catalysts include compounds in which palladium is ligated to phosphines (Pd(PPh3)4). A common derivative is Pd(PPh3)2Cl2, but bidentate ligand catalysts, such as Pd(dppe)Cl, Pd(dppp)Cl2, and Pd(dppf)Cl2 have also been used.[5] The drawback to such catalysts is the need for high loadings of palladium (up to 5 mol %), along with a larger amount of a copper co-catalyst.[5] PdII is often employed as a pre-catalyst since it exhibits greater stability than Pd0 over an extended period of time and can be stored under normal laboratory conditions for months.[8] The Pd II catalyst is reduced to Pd0 in the reaction mixture by either an amine, a phosphine ligand, or a reactant, allowing the reaction to proceed.[9] The oxidation of triphenylphosphine to triphenylphosphine oxide can also lead to the formation of Pd0 in situ when catalysts such as bis(triphenylphosphine)palladium(II) chloride are used.
Copper(I) salts, such as copper iodide, react with the terminal alkyne and produce a copper(I) acetylide, which acts as an activated species for the coupling reactions. Cu(I) is a co-catalyst in the reaction, and is used to increase the rate of the reaction.[4]
Reaction Conditions[edit]
The Sonogashira reaction is typically run under mild conditions. The cross-coupling is carried out at room temperature with a base, typically an amine, such as diethylamine,[3] that also acts as the solvent. Depending on the sp2-carbon halide-or triflate used, these reaction conditions have varying results.
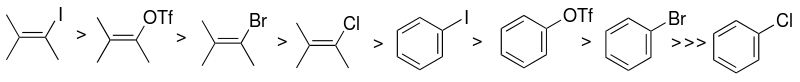
Scope and Limitations[edit]
Copper-Free Reaction[edit]
While a copper co-catalyst is added to the reaction to increase reactivity, the presence of copper can result in the formation of alkyne dimers. This leads to what is known as the Glaser coupling reaction, which is the formation of homocoupling products of acetylene derivatives upon oxidation. As a result, when running a Sonogashira reaction with a copper co-catalyst, it is necessary to run the reaction in an inert atmosphere to avoid the unwanted dimerization. Copper-free variations to the Sonogashira reaction have been developed to avoid the formation of the homocoupling products.[8][10] The exact mechanism by which the copper-free reaction occurs is still under debate.[4]
Catalyst Variations[edit]
Recently, a nickel-catalyzed Sonogashira coupling has been developed which allows for the coupling of non-activated alkyl halides to acetylene without the use of palladium, although a copper co-catalyst is still needed.[11] It has also been reported that gold can be used as a catalyst when it is in the form of Au(I) nanoparticles, which was demonstrated in the coupling of phenylacetylene and iodobenzene with an Au/CeO2 catalyst.[12][13]
Applications in Pharmaceuticals[edit]
The versatility of the Sonogashira reaction makes it a widely used reaction in the synthesis of a variety of compounds. One such pharmaceutical application is in the synthesis of SIB-1508Y, which is more commonly known as Altinicline. Altinicline is a nicotinic acetylcholine receptor agonist that has shown potential in the treatment of Parkinson’s disease, Alzheimer’s disease, Tourette’s syndrome, Schizophrenia, and attention deficit hyperactivity disorder (ADHD).[2][14] As of 2008, Altinicline has undergone Phase II clinical trials.[15][16]

Applications in Natural Products[edit]
Many metabolites found in nature contain alkyne or enyne moieties, and therefore, the Sonogashira reaction has found frequent utility in their syntheses. An example is the synthesis of the benzylisoquinoline alkaloids (+)-(S)-laudanosine and (–)-(S)-xylopinine. The synthesis of these natural products involved the use of Sonogashira cross-coupling to build the carbon backbone of each molecule.[17]

Procedure[edit]
A procedure for the synthesis of 2-chloro-1-decen-3-yne provides typical reaction conditions for a Sonogashira cross-coupling. The coupling of 1-octyne with vinylidene chloride uses tetrakis(triphenylphosphine)palladium(0) (Pd(PPh3)4), n-butylamine, and copper(I) iodide in toluene at 40 °C to form the cross-coupled product, 2-chloro-1-decen-3-yne.[19]
Related Reactions[edit]
- Castro-Stephens coupling
- Heck reaction
- Stille reaction
- Suzuki reaction
- Negishi coupling
- Kumada coupling
References[edit]
- ^ a b Sonogashira, K. (2002), "Development of Pd-Cu catalyzed cross-coupling of terminal acetylenes with sp2-carbon halides", J. Organomet. Chem., 653 (1–2): 46–49, doi:10.1016/s0022-328x(02)01158-0
- ^ a b c King, A. O.; Yasuda, N. (2004), "Palladium-Catalyzed Cross-Coupling Reactions in the Synthesis of Pharmaceuticals Organometallics in Process Chemistry", Top. Organomet. Chem., 6: 205–245, doi:10.1007/b94551
{{citation}}: CS1 maint: multiple names: authors list (link) - ^ a b Sonogashira, K.; Tohda, Y.; Hagihara, N. (1975), "A convenient synthesis of acetylenes: catalytic substitutions of acetylenic hydrogen with bromoalkenes, iodoarenes and bromopyridines", Tetrahedron Lett. , 16 (50): 4467–4470, doi:10.1016/s0040-4039(00)91094-3
{{citation}}: CS1 maint: multiple names: authors list (link) - ^ a b c d e f Chinchilla, R.: Najera, C. (2011), "Recent advances in Sonogashira reactions", Chem. Soc. Rev., 40 (10): 5084–5121, doi:10.1039/c1cs15071e, PMID 21655588
{{citation}}: CS1 maint: multiple names: authors list (link) - ^ a b c d Chinchilla, R.; Najera, C. (2007), "The Sonogashira Reaction: A Booming Methodology in Synthetic Organic Chemistry", Chem. Rev., 107 (3): 874–922, doi:10.1021/cr050992x, PMID 17305399
{{citation}}: CS1 maint: multiple names: authors list (link) - ^ Stambuli, J. P.; Buhl, M.; Hartwig, J. F. (2002), "Synthesis, Characterization, and Reactivity of Monomeric, Arylpalladium Halide Complexes with a Hindered Phosphine as the Only Dative Ligand", J. Am. Chem. Soc., 124 (32): 9346–9347, doi:10.1021/ja0264394, PMID 12167009
{{citation}}: CS1 maint: multiple names: authors list (link) - ^ Amatore, C.; Jutand, A. (2000), "Anionic Pd(0) and Pd(II) Intermediates in Palladium-Catalyzed Heck and Cross-Coupling Reactions", Acc. Chem. Res., 33 (5): 314–321, doi:10.1021/ar980063a, PMID 10813876
{{citation}}: CS1 maint: multiple names: authors list (link) - ^ a b Bohm, V. P. W.; Herrmann, W. A. (2000), "A Copper-Free Procedure for the Palladium-Catalyzed Sonogashira Reaction of Aryl Bromides with Terminal Alkynes at Room Temperature", Eur. J. Org. Chem. , 200 (22): 3679–3681, doi:10.1002/1099-0690(200011)2000:22<3679::aid-ejoc3679>3.0.co;2-x
{{citation}}: CS1 maint: multiple names: authors list (link) - ^ Yin, L.; Liebscher, J. (2006), "Carbon-Carbon Coupling Reactions Catalyzed by Heterogeneous Palladium Catalysts", Chem. Rev., 107 (1): 133–173, doi:10.1021/cr0505674, PMID 17212474
{{citation}}: CS1 maint: multiple names: authors list (link) - ^ Mery, D.; Heuze, K.; Astruc, D. (2003), "A very efficient, copper-free palladium catalyst for the Sonogashira reaction with aryl halides", Chem. Commun., 15 (15): 1934–1935, doi:10.1039/B305391C, PMID 12932040
{{citation}}: CS1 maint: multiple names: authors list (link) - ^ Vechorkin, O.; Barmaz, D.; Proust, V., Hu, X. (2009), "Ni-Catalyzed Sonogashira Coupling of Nonactivated Alkyl Halides: Orthogonal Functionalization of Alkyl Iodides, Bromides, and Chlorides", J. Am. Chem. Soc., 131 (34): 12078–12079, doi:10.1021/ja906040t, PMID 19670863
{{citation}}: CS1 maint: multiple names: authors list (link) - ^ Gonzalez-Arallano, C.; Abad, A.; Corma, A.; Garcia, H.; Iglesias, M.; Sanchez, F. (2007), "Catalysis by Gold(I) and Gold(III): A Parallelism between Homo- and Heterogeneous Catalysts for Copper-Free Sonogashira Cross-Coupling Reactions", Angew. Chem. Int. Ed., 46 (9): 1536–1538, doi:10.1002/anie.200604746, PMID 17226890
{{citation}}: CS1 maint: multiple names: authors list (link) - ^ Corma, A.; Juarez, R.; Boronat, M.; Sanchez, F.; Iglesias, M.; Garcia, H. (2011), "Gold catalyzes the Sonogashira coupling reaction without the requirement of palladium impurities", Chem. Commun., 47 (5): 1446–1448, doi:10.1039/C0CC04564K, PMID 21183985
{{citation}}: CS1 maint: multiple names: authors list (link) - ^ Bleicher, L. S.; Cosford, N. D. P.; Herbaut, A.; McCallum, J. S.; McDonald, I. A. (1998), "A Practical and Efficient Synthesis of the Selective Neuronal Acetylcholine-Gated Ion Channel Agonist (S)-(-)-5-Ethynyl-3-(1-methyl-2-pyrrolidinyl)pyridine Maleate (SIB-1508Y)", J. Org. Chem., 63: 1109–1118, doi:10.1021/jo971572d
{{citation}}: CS1 maint: multiple names: authors list (link) - ^ Wang, David X. (1 September 1998). "Structure-activity relationships for nicotine analogs comparing competition for [3H]nicotine binding and psychotropic potency". Drug Development Research. 45 (1): 10–16. doi:10.1002/(SICI)1098-2299(199809)45:1<10::AID-DDR2>3.0.CO;2-G.
{{cite journal}}: Unknown parameter|coauthors=ignored (|author=suggested) (help) - ^ "Randomized placebo-controlled study of the nicotinic agonist SIB-1508Y in Parkinson disease". Neurology. 66 (3): 408–410. 14 February 2006. doi:10.1212/01.wnl.0000196466.99381.5c. PMID 16476941. S2CID 31720763.
- ^ Mujahidin, Didin (1 July 2005). "Enantioselective Synthesis of (+)-(S)-Laudanosine and (-)-(S)-Xylopinine". European Journal of Organic Chemistry. 2005 (13): 2689–2693. doi:10.1002/ejoc.200500095.
{{cite journal}}: Unknown parameter|coauthors=ignored (|author=suggested) (help) - ^ Mujahidin, Didin (1 July 2005). "Enantioselective Synthesis of (+)-(S)-Laudanosine and (-)-(S)-Xylopinine". European Journal of Organic Chemistry. 2005 (13): 2689–2693. doi:10.1002/ejoc.200500095.
{{cite journal}}: Unknown parameter|coauthors=ignored (|author=suggested) (help) - ^ Kohnen, A. L; Danheiser, R. L. (2007), "Synthesis of Terminal 1,3-Diynes Via Sonogashira Coupling of Vinylidene Chloride Followed by Elimination. Preparation of 1,3-Decadiyne" (PDF), Org. Syn., 84: 77–87, doi:10.1002/0471264229.os084.08, ISBN 978-0471264224, PMC 2901882, PMID 20628544
{{citation}}: CS1 maint: multiple names: authors list (link)

