User:Hsrinimukesh3/Hereditary diffuse leukoencephalopathy with spheroids (HDLS)
| This user page or section is in a state of significant expansion or restructuring. You are welcome to assist in its construction by editing it as well. If this user page has not been edited in several days, please remove this template. If you are the editor who added this template and you are actively editing, please be sure to replace this template with {{in use}} during the active editing session. Click on the link for template parameters to use.
This page was last edited by Jonesey95 (talk | contribs) 6 months ago. (Update timer) |
| Hsrinimukesh3/Hereditary diffuse leukoencephalopathy with spheroids (HDLS) |
|---|
Hereditary diffuse leukoencephalopathy with spheroids (HDLS) is a rare adult onset autosomal dominant disorder characterized by cerebral white matter degeneration with axonal spheroids leading to progressive cognitive and motor dysfunction. It is believed that the disease arises from primary disruption of axonal integrity, neuroaxonal damage, and focal axonal spheroids leading to demyelination. Specifically, shear stress damages the axons, causing them to swell to spherical clumps. The blockage of axoplasmic transport resulting from these axonal spheroids can cause trauma, stroke, and many other symptoms seen in degenerative diseases.[1] It is uncertain whether demyelination occurs prior to the axonal spheroids to trigger autonomous neurodegeneration.[2] It is commonly mistaken for Alzheimer's disease, Frontotemporal dementia, Atypical Parkinsonism, Multiple sclerosis, and Corticobasal degeneration.[3]
Classification[edit]
HDLS falls under brain white matter diseases called Leukoencephalopathies that are characterized by some degree of white matter dysfunction. HDLS specifically experiences white matter lesions as well as abnormalities in myelin sheath formation, where the causative influences are being continually explored past the recent genetic findings. Studies by Dr. Sundal from Sweden show that a risk allele in Caucasians may be causative because cases identified have thus far been among large Caucasian families. An average profile from current studies show that the median onset age for HDLS patients is 44 with a disease duration of 48 and mean age of death at 48.[2] Through the study of numerous kindred, it was found that the disease did not occur among just males or just females, but rather was evenly distributed indicative of an autosomal relationship rather than sex-linked. It was also noticed that the HDLS cases did not skip generations as would be noticed with recessive allelic characteristics, and as such has been labeled dominant.[2]
Clinical Symptoms & Misdiagnoses[edit]

With symptoms of personality changes, behavioral changes, dementia, depression, and epilepsy, HDLS has been commonly misdiagnosed for many other diseases. [4]Dementia or frontotemporal dementia for example has commonly steered some diagnoses toward Alzheimer’s disease when it was actually HDLS. HDLS commonly manifests with neuropsychiatric symptoms, progressing to dementia, and after a few years shows motor dysfunction. Eventually patients become wheelchair-bound or bedridden.[3]
With the difficulty of identifying cases as exclusively HDLS, researchers have looked into the exclusive traits of common adult onset leukodystrophies such as Metachromatic leukodystrophy (MLD), Krabbe disease, and X-linked adrenoleukosystrophy (X-ADL) to eliminate them during differential diagnoses.[2]
| Disease | Exclusive Trait |
|---|---|
| MLD | Accumulation of metachromatic material in white matter |
| Krabbe Disease | Presence of globoid cells derived from microglia which have multiple nuclei |
| X-ALD | Predominant parieto-occipital white matter abnormality |
| Vanishing White Matter (VWM) Disease |
|
| Nasu-Hakola |
|
Neuropsychiatric Symptoms[edit]
Many neuropsychiatric symptoms have been identified by genealogical studies. These include severe depression and anxiety that have been identified in about 70% of HDLS families verging on suicidal tendencies and substance abuse such as alcoholism. Additionally, patients may exhibit disorientation, confusion, agitation, irritability, aggressiveness, an altered mental state, the loss of the ability to execute learned movements (Apraxia), or the inability to speak (Mutism). [3]
Motor Impairment[edit]
Persons with HDLS can suffer from tremors, decreased body movement, unsteadiness (Parkinsonism, muscles on one side of the body in constant contraction (Spastic Hemiparesis), impairment in motor and sensory function in the lower extremities (Paraparesis), paralysis resulting in partial or total loss of all extremities and torso (Tetraparesis), and the lack of voluntary coordination of muscle movements (Ataxia). [3]
History & Origination[edit]
This disease was first described in 1984 by Axelsson et al in a large Swedish pedigree.[5] Dr. Dennis W. Dickson, who has taken on the pathological study component of investigating HDLS, gained his motivation in the early 90s when he was covering neuropathology in New York on cases that had previously been identified. This curiosity was further heightened in 1997 at the Mayo Clinic when Dr. Zbigniew K. Wszolek came across cases with similar symptoms. Since the establishment of an international consortium in 2005 by Dr. Wzolek, several families have been identified with HDLS through the Mayo Clinic.[2]
Diseases of the same disease spectrum as HDLS include Nasu-Hakola and POLD,a type of leukodystrophy called pigmentary orthochromatic leukodystrophy. [3] Nasu-Hakola causes development of bone disease through involvement of the same colony stimulating factor (CSF) signaling cascade as identified in HDLS. [6] While different gene mutations occur with the pathway, both present white matter axonal spheroids. Current researchers in the field believe that deeper analysis of the two disorders in comparison could lead to a better understanding of these two rare complications from a genetic standpoint. POLD exhibits noninflammatory demyelination of axons with initial symptoms of euphoria, apathy, headache, and executive dysfunction. While HDLS has been labeled as autosomal dominant, POLD has been labeled as autosomal recessive. [7]
Pathology[edit]
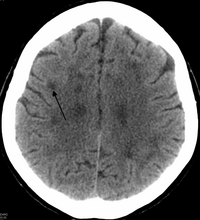
There is enlargement of the lateral ventricles and marked attenuation of cerebral white matter.[8] The loss of white matter is caused by myelin loss. These changes are associated with diffuse gliosis, moderate loss of axons and many axonal spheroids.
Spheroids are thin and discontinuous or lack myelin sheaths entirely.[1] Macrophages with non-metachromatic lipid granules are common. In severely degenerated areas there are lipid-laden macrophages with an aggregation of membranous bodies and large, reactive astrocytes filled with glial fibrils.[1] In studied cases, it has been shown that white matter abnormalities have been confined to the cerebellum while avoiding major fiber tracts of the nervous system. White matter tracts were unaffected in the brainstem.[2]
The pathology of this disease resembles that of Nasu-Hakola disease (polycystic lipomembranous osteodysplasia with sclerosing leukoencephalopathy).[9] Nasu-Hakola disease appears to be caused by mutations in the TYRO protein tyrosine kinase-binding protein and the Triggering receptor expressed on myeloid cells 2 protein.
Genetics[edit]
The cause of this disease appears to be due to mutations in the colony stimulating factor 1 receptor (CSF1R).[4]The mutations are concentrated in tyrosine kinase domain (TKD) of the protein. Mutations were mainly found along exons 12-22 of the intracellular TKD, detailing 10 missense mutations and a single codon deletion. Additionally three splice site mutations were identified that caused an in-frame deletion of an exon leading to the removal of more than 40 amino acids in the TKD.[4] This determination has arisen from 14 HDLS families confirming mutations in this gene. The receptor protein primarily functions in regulation, survival, proliferation, and differention of microglial cells.[10] The process from the microglial function of CSF1R to the axonal spheroid formation remains unknown, leading to work needed to better understand this pathogenesis.
Research[edit]
Current research includes the study to investigate stimulated microglial function. This work would further clarify whether the disease is glial degenerative. For such a study, microglial cells from HDLS kindred will be analyzed and compared to normal microglial cells on the basis of differences in mutation occurrences and growth factor expression.
Clinical and Genealogic Studies[edit]

To gain a better understanding of the disease state, researchers have retrospectively reviewed medical records of probands and were also assessed through numerous other examinations. Blood samples were collected from the families of these probands for genetic testing. These family members were assessed using their standard medical history, on their progression of Parkinson's like symptoms (Unified Parkinson's Disease Rating Scale), and on their progression of cognitive impairment such as dementia (Folstein Test).[2]
Neuroimaging[edit]
Standard MRI scans are performed on 1.5 Tesla scanners with 5mm thickness and 5mm spacing to screen for white matter lesions in identified families. If signal intensities of the MRI scans are higher in white matter regions than in grey matter regions, the patient is marked as pathologic.[2]
Pathology[edit]
Paraffin blocks from autopsies and glass slides from brain biopsies were performed on identified probands by the Florida location of the Mayo Clinic where sections of the neocortex, hippocampus, hypothalmus, basal ganglia, thalamus, midbrain, pons, cerebellum, and spinal cord were examined.[2][11] H&E staining was done on these samples as well as immunohistory for ubiquitin among several other techniques to characterize white matter pathology.[8] With a similar pathology to POLD, HDLS is commonly grouped with POLD under the title Adult-Onset Leukoencehalopathy with Axonal Spheroids and Pigmented Glia (ALSP) so as to give these individually under-recognized conditions heightened attention.[3]
See also[edit]
- Colony Stimulating Factor 1 Receptor
- Neurodegeneration
- Leukoencephalopathy with vanishing white matter
References[edit]
- ^ a b c Lin, W. L., Wszolek, Z. K., & Dickson, D. W. (2010). Hereditary diffuse leukoencephalopathy with spheroids: ultrastructural and immunoelectron microscopic studies. Int J Clin Exp Pathol, 3(7), 665-674.
- ^ a b c d e f g h i j Sundal, C., Lash, J., Aasly, J., Oygarden, S., Roeber, S., Kretzschman, H., . . . Wszolek, Z. K. (2012). Hereditary diffuse leukoencephalopathy with axonal spheroids (HDLS): a misdiagnosed disease entity. J Neurol Sci, 314(1-2), 130-137. doi: 10.1016/j.jns.2011.10.006
- ^ a b c d e f Wider, C., Van Gerpen, J. A., DeArmond, S., Shuster, E. A., Dickson, D. W., & Wszolek, Z. K. (2009). Leukoencephalopathy with spheroids (HDLS) and pigmentary leukodystrophy (POLD): a single entity? Neurology, 72(22), 1953-1959. doi: 10.1212/WNL.0b013e3181a826c0
- ^ a b c Rademakers, R., Baker, M., Nicholson, A., Rutherford, N., Finch, N., Soto-Ortolaza, A., . . . Wszolek, Z. (2012). Mutations in the colony stimulating factor 1 receptor (CSF1R) cause hereditary diffuse leukoencephalopathy with spheroids. Movement Disorders, 27, S399-S400.
- ^ Axelsson, R., Roytta, M., Sourander, P., Akesson, H. O., & Andersen, O. (1984). Hereditary diffuse leucoencephalopathy with spheroids. Acta Psychiatr Scand Suppl, 314, 1-65.
- ^ Paloneva, J., Mandelin, J., Kiialainen, A., Böhling, T., Prudlo, J., Hakola, P., . . . Peltonen, L. (2003). DAP12/TREM2 deficiency results in impaired osteoclast differentiation and osteoporotic features. The Journal of experimental medicine, 198(4), 669-675.
- ^ Knaap, Marjo S., & Valk, Jaap. (2005). Pigmentary Orthochromatic Leukodystrophy Magnetic Resonance of Myelination and Myelin Disorders (pp. 557-558): Springer Berlin Heidelberg.
- ^ a b Baba, Y., Ghetti, B., Baker, M. C., Uitti, R. J., Hutton, M. L., Yamaguchi, K., . . . Wszolek, Z. K. (2006). Hereditary diffuse leukoencephalopathy with spheroids: clinical, pathologic and genetic studies of a new kindred. Acta Neuropathol, 111(4), 300-311. doi: 10.1007/s00401-006-0046-z
- ^ Hancock, N., Poon, M., Taylor, B., & McLean, C. (2003). Hereditary diffuse leucoencephalopathy with spheroids. J Neurol Neurosurg Psychiatry, 74(9), 1345-1347.
- ^ Kinoshita, M., Yoshida, K., Oyanagi, K., Hashimoto, T., & Ikeda, S. (2012). Hereditary diffuse leukoencephalopathy with axonal spheroids caused by R782H mutation in CSF1R: Case report. Journal of the Neurological Sciences, 318(1-2), 115-118. doi: 10.1016/j.jns.2012.03.012
- ^ Van Gerpen, J. A., Wider, C., Broderick, D. F., Dickson, D. W., Brown, L. A., & Wszolek, Z. K. (2008). Insights into the dynamics of hereditary diffuse leukoencephalopathy with axonal spheroids. Neurology, 71(12), 925-929. doi: 10.1212/01.wnl.0000325916.30701.21
