User:Egs1230/sandbox/chem275
| |||
| Names | |||
|---|---|---|---|
| IUPAC name
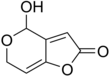
4-hydroxy-4H-furo[3,2-c]pyran-2(6H)-one
| |||
| Other names
2-Hydroxy-3,7-dioxabicyclo[4.3.0]nona-5,9-dien-8-one
Clairformin Claviform Expansine Clavacin Clavatin Expansin Gigantin Leucopin Patuline | |||
| Identifiers | |||
3D model (JSmol)
|
|||
| ChEBI | |||
| ChEMBL | |||
| ChemSpider | |||
| EC Number |
| ||
| KEGG | |||
PubChem CID
|
|||
| UNII | |||
| |||
| |||
| Properties | |||
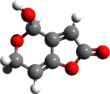
| C7H6O4 | |||
| Molar mass | 154.12 g/mol | ||
| Appearance | Compact prisms | ||
| Density | 1.52 g/ml | ||
| Melting point | 110 °C (230 °F; 383 K) | ||
| Soluble | |||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |||
Patulin is a mycotoxin known as a polyketide produced in a variety of molds such as Aspergillus, Penicillium, and Byssochlamys with Penicillium expansum as a primary producer. Toxicity can cause symptoms such as nausea, vomiting, and intestinal hemorrhaging.[2] Patulin is found mainly on moldy organic matter, particularly fruits and vegetables, with apples being a main source of patulin. Health organizations around the world regulate the levels of patulin that can be in foods with dosages ranging from 10 ug/kg bw/d in infant food to 50 ug/kg bw/d for all other foods.[3]
Biosynthesis and reactivity[edit]
Biosynthesis[edit]
The biosynthesis of patulin begins with acetyl-CoA and 3 malonyl-CoA, which is then processed by 6-methylsalicylic acid synthase into 6-methylsalicylic acid.[3][4] The entire biosynthesis, omitting metabolites, is outlined here.[3][4]

Like other filamentous fungi, the genes encoding for secondary metabolite production of patulin are putatively grouped into gene clusters, including a gene for 6-MSAS and isoepoxydon dehydrogenase (IDH), which are involved directly with synthesis.[5][6][7][8] Specifically, there are fifteen genes within the patulin gene cluster, some with identified functions and others with unknown function.[9] Processing via the cytochrome P450 (CYP450) derivatives CYP619C3 and CYP619C2, hydroxylates the m-cresol to m-hydroxybenzyl via 619C3, followed by the conversion of m-hydroxybenzyl to gentisyl alcohol via 619C2, both of which are key steps in the biosynthetic pathway.
Reactivity[edit]
Patulin is unstable in the presence of sulfur dioxide, however, studies describing its effectiveness at reducing the amount of patulin present show varying results from 90% elimination to 12% elimination depending on protocol followed.[10] The sulfur dioxide proceeds through a two-step mechanism wherein the sulfur dioxide will add to the hemiacetal ring, which forms a carbonyl hydroxysulfonate. This product will then be irreversibly inactivated by the opening of the lactone ring.[11]
Patulin may primarily exhibit toxic activity via covalent binding of thiol containing molecules, forming thiol-thiol, thiol-amine, and amine-amine inter- and intramolecular crosslinks.[12] Specifically, it has affinity for cysteine, lysine, and histidine containing molecules.[13] Patulin has also shown evidence of upregulating proteosome activity, which can have proteotoxic effects.[13][12]
In addition, patulin concurrently reduces the levels of antioxidants within the cell, such as glutathione, while the levels of oxidative stress markers increased. Patulin may play a role in increasing reactive oxygen species (ROS) within a cell, which may lead to many outcomes, including mitochondrial dysfunction, autophagy, and apoptosis by direct interaction with the PI3K/AKT/mTOR pathway.
Inhibitors of Patulin[edit]
Blue LED light negatively impacted patulin production in fungi by down-regulating the enzymatic activity of enzymes produced by the PatA, PatE, and PatN genes, which encode for an acetate transporter, a glucose-methanol-choline oxidoreductase, and isoepoxydon dehydrogenase respectively.[14] All of these are key in steps 5, 7, and 10 of the patulin biosynthesis pathway.[15]
Another path is the biological approach, using neem leaf extract to inhibit patulin production. While the mechanism is unknown, patulin production was reduced nearly 100% in P.expansum and could provide a way to control production without the use of harsh chemicals.[16]Similarly, use of cinnamon oil to inhibit the growth of P.expansum negatively impacted the amount of patulin produced by the mold in a dose dependent manner.[17]The cinnamon oil also negatively impacted the carbohydrate metabolic process by modifying the activity of crucial enzymes such as ß-glucosidase, α-ketoglutarate dehydrogenase, hexokinase, 6-phosphofructokinase, pyruvate kinase, and glucose 6-phosphate dehydrogenase.
Additionally, targeting enzymes in the biosynthesis pathway may prove fruitful, particularly the enzyme produced by the PatE gene, the GMC oxidoreductase, that is responsible for the last step in the biosynthesis of patulin. This is an area of developing research but compounds such as umbelliferone and meticrane are being studied in order to revisit the pharmacologic intervention into patulin inhibition.[18][19]
Uses[edit]
Patulin was initially isolated for use as a broad spectrum antibiotic against gram positive and gram negative bacteria that cause the common cold due to its bacteriostatic activity.[20] However, testing was discontinued due to ineffectiveness and toxicity. Afterwards, there was no established use for patulin again outside of use as a standard when testing other mycotoxins.[21] Recently, there has been a resurgence in the interest of patulin as an anticancer drug. Recent studies have used patulin in combinatorial therapies to fight ovarian cancer. In combination with cisplatin and emetine, a synergistic effect took place in a dose dependent manner.[22] Due to its well known toxicity, the use of patulin is worrisome, however, short term use of patulin does not cause toxicity and is excreted quickly by the body. Along that line, patulin is classified as a 'Class 3' agent by the International Agency for Research on Cancer, concluding that patulin is not carcinogenic.[23]
Sources of exposure[edit]
Frequently, patulin is found in apples and apple products such as juices, jams, and ciders. It has also been detected in other fruits including cherries, blueberries, plums, bananas, strawberries, and grapes.[24] Fungal growth leading to patulin production is most common on damaged fruits.[25] Patulin has also been detected in grains like barley, wheat, corn and their processed products as well as in shellfish.,[24][26] Dietary intake of patulin from apple juice has been estimated at between 0.03 and 0.26 μg/kg bw/day in various age groups and populations.[27] Content of patulin in apple juice is estimated to be less than 10–15μg/L.[27] A number of studies have looked into comparisons of organic vs conventional harvest of apples and levels of patulin contamination.[28][29][30] For example, one study showed 0.9% of children drinking organic apple juice exceeded the tolerable daily intake (TDI) for patulin.[31] A recent article described detection of patulin in marine strains of Penicillium, indicating a potential risk in shellfish consumption.[26]
Toxicity[edit]
A subacute rodent NOAEL of 43 μg/kg body weight as well as genotoxicity studies were primarily the cause for setting limits for patulin exposure, although a range of other types of toxicity also exist.[32]
While not a particularly potent toxin, patulin is genotoxic. Some theorize that it may be a carcinogen, although animal studies have remained inconclusive.[33] Patulin has shown antimicrobial properties against some microorganisms.[1] Several countries have instituted patulin restrictions in apple products. The World Health Organization recommends a maximum concentration of 50 µg/L in apple juice.[34] In the European Union, the limit is also set at 50 micrograms per kilogram (µg/kg) in apple juice and cider, at 25 µg/kg in solid apple products, and at 10 µg/kg in products for infants and young children. These limits came into force on 1 November 2003.[35]
Acute[edit]
Patulin is toxic primarily through affinity to sulfhydryl groups (SH), which results in inhibition of enzymes. Oral LD50 in rodent models have ranged between 20 and 100 mg/kg.[32] In poultry, the oral LD50 range was reported between 50 and 170 mg/kg.[36] Other routes of exposure are more toxic, yet less likely to occur. Major acute toxicity findings include gastrointestinal problems, neurotoxicity (i.e. convulsions), pulmonary congestion, and edema.[32]
Subacute[edit]
Studies in rats showed decreased weight, and gastric, intestinal, and renal function changes, while repetitive doses lead to neurotoxicity. Reproductive toxicity in males was also reported.[36] A NOAEL in rodents was observed at 43μg/kg bw.[32]
Genotoxicity[edit]
WHO concluded that patulin is genotoxic based on variable genotoxicity data, however it is considered a group 3 carcinogen by the International Agency for Research on Cancer (IARC) since data was inconclusive.[32]
Reproduction studies[edit]
Patulin decreased sperm count and altered sperm morphology in the rat.[37] Also, it resulted in abortion of F1 litters in rats and mice after i.p. injection.[36] Embryotoxicity and teratogenicity were also reported in chick eggs.[36]
Immunotoxicity[edit]
Patulin was found to be immunotoxic in a number of animal and even human studies. Reduced cytokine secretion, oxidative burst in macrophages, increased splenic T lymphocytes, and increased neutrophil numbers are a few endpoints noticed.[36] However, dietary relevant exposure would not be likely to alter immune response.[24]
Human health[edit]
Although there are only very few reported cases and epidemiological data, the FDA has set an action limit of 50 ppb in cider due to its potential carcinogenicity and other reported adverse effects.[32] In humans, it was tested as an antibiotic intranasally for use against the common cold with few significant adverse effects, yet also had negligible or no beneficial effect.[38]
Risk management and regulations[edit]
Ways of regulating patulin production may lie in environmental conditions, with water, temperature, pH, and nitrogen content being crucial for the formation of patulin.[39] The most effective way of regulating patulin on an industrial scale is to improve agricultural practices. The four most important concepts in the prevention of patulin producing fungi include 1) moisture control, 2) quality assessment, 3) manufacturing practices, and 4) adherence to food safety and inspection guidelines, such as those outlined in Hazard Analysis and Critical Control Point (HACCP).[40][41]
US
The provisional tolerable daily intake (PTDI) for patulin was set at 0.43 µg/kg bw by the FDA[32] based on a NOAEL of 0.3 mg/kg bw per week.[32] Monte Carlo analysis was done on apple juice to compare exposure and the PTDI. Without controls or an action limit, the 90th percentile of consumers would not be above the PTDI. However, the concentration in children 1–2 years old would be three times as high as the PDTI, hence an action limit of 50 µg/kg.[32]
WHO
The World Health Organization recommends a maximum concentration of 50 µg/L in apple juice.[34]
EU
The European Union (EU) has set a maximum limit of 50μg/kg on fruit juices and drinks, while solid apple products have a limit of 25μg/kg. For certain foods intended for infants, an even lower limit of 10μg/kg is observed.
To test for patulin contamination, a variety of methods and sample preparation methods have been employed, including thin layer chromatography (TLC), gas chromatography (GC), high-performance liquid chromatography (HPLC), and capillary electrophoresis.[42]
References[edit]
- ^ a b Merck Index, 11th Edition, 7002
- ^ Diao, Enjie; Ma, Kun; Zhang, Hui; Xie, Peng; Qian, Shiquan; Song, Huwei; Mao, Ruifeng; Zhang, Liming (16 September 2021). "Thermal Stability and Degradation Kinetics of Patulin in Highly Acidic Conditions: Impact of Cysteine". Toxins. 13 (9): 662. doi:10.3390/toxins13090662.
{{cite journal}}: CS1 maint: unflagged free DOI (link) - ^ a b c Li, Boqiang; Chen, Yong; Zhang, Zhanquan; Qin, Guozheng; Chen, Tong; Tian, Shiping (November 2020). "Molecular basis and regulation of pathogenicity and patulin biosynthesis in Penicillium expansum". Comprehensive Reviews in Food Science and Food Safety. 19 (6): 3416–3438. doi:10.1111/1541-4337.12612.
- ^ a b Artigot, Marie Pierre; Loiseau, Nicolas; Laffitte, Joelle; Mas-Reguieg, Lina; Tadrist, Souria; Oswald, Isabelle P.; Puel, Olivier (1 May 2009). "Molecular cloning and functional characterization of two CYP619 cytochrome P450s involved in biosynthesis of patulin in Aspergillus clavatus". Microbiology. 155 (5): 1738–1747. doi:10.1099/mic.0.024836-0.
- ^ Light, Robley J. (November 1969). "6-Methylsalicylic acid decarboxylase from Penicillium patulum". Biochimica et Biophysica Acta (BBA) - Enzymology. 191 (2): 430–438. doi:10.1016/0005-2744(69)90262-9.
- ^ Sekiguchi, Junichi; Gaucher, G. Maurice (1 August 1979). "Patulin biosynthesis: the metabolism of phyllostine and isoepoxydon by cell-free preparations from Pencillium urticae". Canadian Journal of Microbiology. 25 (8): 881–887. doi:10.1139/m79-131.
- ^ Murphy, Gillian; Vogel, Gunter; Krippahl, Gunther; Lynen, Feodor (November 1974). "Patulin Biosynthesis: The Role of Mixed-Function Oxidases in the Hydroxylation of m-Cresol". European Journal of Biochemistry. 49 (2): 443–455. doi:10.1111/j.1432-1033.1974.tb03849.x.
- ^ Murphy, Gillian; Lynen, Feodor (October 1975). "Patulin Biosynthesis: The Metabolism of m-Hydroxybenzyl Alcohol and m-Hydroxybenzaldehyde by Particulate Preparations from Penicillium patulum". European Journal of Biochemistry. 58 (2): 467–475. doi:10.1111/j.1432-1033.1975.tb02394.x.
- ^ "BGC0000120". mibig.secondarymetabolites.org.
- ^ Moake, Matthew M.; Padilla-Zakour, Olga I.; Worobo, Randy W. (January 2005). "Comprehensive Review of Patulin Control Methods in Foods". Comprehensive Reviews in Food Science and Food Safety. 4 (1): 8–21. doi:10.1111/j.1541-4337.2005.tb00068.x.
- ^ Burroughs, LF (January 1977). "Stability of patulin to sulfur dioxide and to yeast fermentation". Journal - Association of Official Analytical Chemists. 60 (1): 100–3. PMID 319091.
- ^ a b Guerra-Moreno, Angel; Hanna, John (July 2017). "Induction of proteotoxic stress by the mycotoxin patulin". Toxicology Letters. 276: 85–91. doi:10.1016/j.toxlet.2017.05.015.
- ^ a b Fliege, Ralph; Metzler, Manfred (November 1999). "The mycotoxin patulin induces intra- and intermolecular protein crosslinks in vitro involving cysteine, lysine, and histidine side chains, and α-amino groups". Chemico-Biological Interactions. 123 (2): 85–103. doi:10.1016/S0009-2797(99)00123-4.
- ^ Zhu, Ruiyu; Wang, Weilun; Luo, Zisheng; Lin, Haiyan; Li, Yong; Lu, Weiqiang; Xu, Zimu; Cai, Chenggang; Hu, Shuheng (June 2023). "Blue LED light treatment inhibits virulence and patulin biosynthesis in Penicillium expansum". Postharvest Biology and Technology. 200: 112340. doi:10.1016/j.postharvbio.2023.112340.
- ^ Li, Boqiang; Chen, Yong; Zhang, Zhanquan; Qin, Guozheng; Chen, Tong; Tian, Shiping (November 2020). "Molecular basis and regulation of pathogenicity and patulin biosynthesis in Penicillium expansum". Comprehensive Reviews in Food Science and Food Safety. 19 (6): 3416–3438. doi:10.1111/1541-4337.12612.
- ^ Aparecida Galerani Mossini, Simone; De Oliveira, Kesia Pires; Kemmelmeier, Carlos (April 2004). "Inhibition of patulin production byPenicillium expansum cultured with neem (Azadirachta indica) leaf extracts". Journal of Basic Microbiology. 44 (2): 106–113. doi:10.1002/jobm.200310332.
- ^ Lai, Tongfei; Sun, Yangying; Liu, Yaoyao; Li, Ran; Chen, Yuanzhi; Zhou, Ting (9 February 2021). "Cinnamon Oil Inhibits Penicillium expansum Growth by Disturbing the Carbohydrate Metabolic Process". Journal of Fungi. 7 (2): 123. doi:10.3390/jof7020123.
{{cite journal}}: CS1 maint: unflagged free DOI (link) - ^ Tragni, Vincenzo; Cotugno, Pietro; De Grassi, Anna; Cavalluzzi, Maria Maddalena; Mincuzzi, Annamaria; Lentini, Giovanni; Sanzani, Simona Marianna; Ippolito, Antonio; Pierri, Ciro Leonardo (31 December 2020). "Targeting Penicillium expansum GMC Oxidoreductase with High Affinity Small Molecules for Reducing Patulin Production". Biology. 10 (1): 21. doi:10.3390/biology10010021.
{{cite journal}}: CS1 maint: unflagged free DOI (link) - ^ Sanzani, Simona M.; De Girolamo, Annalisa; Schena, Leonardo; Solfrizzo, Michele; Ippolito, Antonio; Visconti, Angelo (January 2009). "Control of Penicillium expansum and patulin accumulation on apples by quercetin and umbelliferone". European Food Research and Technology. 228 (3): 381–389. doi:10.1007/s00217-008-0944-5.
- ^ Hamilton, E.I. (August 1979). "Evaluation of the carcinogenic risk of chemicals to humans". Science of The Total Environment. 12 (3): 289–290. doi:10.1016/0048-9697(79)90093-7.
- ^ Hamilton, E.I. (August 1979). "Evaluation of the carcinogenic risk of chemicals to humans". Science of The Total Environment. 12 (3): 289–290. doi:10.1016/0048-9697(79)90093-7.
- ^ Alam, Md Nur; Yu, Jun Qing; Beale, Philip; Huq, Fazlul (June 2020). "Cisplatin in combination with emetine and patulin showed dose and sequence dependent synergism against ovarian cancer". Synergy. 10: 100060. doi:10.1016/j.synres.2019.100060.
- ^ Hamilton, E.I. (August 1979). "Evaluation of the carcinogenic risk of chemicals to humans". Science of The Total Environment. 12 (3): 289–290. doi:10.1016/0048-9697(79)90093-7.
- ^ a b c Cite error: The named reference
Llewellynwas invoked but never defined (see the help page). - ^ "Patulin". Archived from the original on 2013-10-18. Retrieved 2013-11-25.
- ^ a b Pouchous et al. Shellfish
- ^ a b Wouters, FA, and Speijers, GJA. JECFA Monograph on Patulin. World Health Organization Food Additives Series 35 (http://www.inchem.org/documents/jecfa/jecmono/v26je10.htm)
- ^ Pique, E., et al. Occurrence of patulin in organic and conventional apple juice. Risk Assessment. Recent Advances in Pharmaceutical Sciences, III, 2013: 131–144.
- ^ Piemontese, L.; Solfrizzo, M.; Visconti, A. (2005-05-01). "Occurrence of patulin in conventional and organic fruit products in Italy and subsequent exposure assessment". Food Additives and Contaminants. 22 (5): 437–442. doi:10.1080/02652030500073550. ISSN 0265-203X. PMID 16019815. S2CID 31155096.
- ^ Piqué, E; Vargas-Murga, L; Gómez-Catalán, J; Lapuente, Jd; Llobet, JM (October 2013). "Occurrence of patulin in organic and conventional apple-based food marketed in Catalonia and exposure assessment". Food and Chemical Toxicology. 60: 199–204. doi:10.1016/j.fct.2013.07.052. PMID 23900007.
- ^ Beark et al 2007
- ^ a b c d e f g h i Cite error: The named reference
ucm212520was invoked but never defined (see the help page). - ^ "Patulin: a Mycotoxin in Apples". Perishables Handling Quarterly (91): 5. August 1997
- ^ a b "Foodborne hazards (World Health Organization". Retrieved 2007-01-22.
- ^ Patulin information leaf from Fermentek
- ^ a b c d e Cite error: The named reference
Puelwas invoked but never defined (see the help page). - ^ Selmanoglu, G (2006). "Evaluation of the reproductive toxicity of patulin in growing male rats". Food Chem. Toxicol. 44 (12): 2019–2024. doi:10.1016/j.fct.2006.06.022. PMID 16905234.
- ^ Cite error: The named reference
MRCwas invoked but never defined (see the help page). - ^ Moake, Matthew M.; Padilla-Zakour, Olga I.; Worobo, Randy W. (January 2005). "Comprehensive Review of Patulin Control Methods in Foods". Comprehensive Reviews in Food Science and Food Safety. 4 (1): 8–21. doi:10.1111/j.1541-4337.2005.tb00068.x.
- ^ Nutrition, Center for Food Safety and Applied (14 September 2022). "Guidance for Industry: Juice HACCP and the FDA Food Safety Modernization Act". U.S. Food and Drug Administration.
- ^ Kabak, Bulent (2010). "Prevention and Management of Mycotoxins in Food and Feed". Mycotoxins in Food, Feed and Bioweapons. Springer: 201–227. doi:10.1007/978-3-642-00725-5_13.
- ^ Variability and uncertainty assessment of patulin exposure for preschool children in Flanders
External links[edit]
- Patulin Archived 2017-12-19 at the Wayback Machine, Food Safety Watch