User:David.Throop/Eicosanoid

In biochemistry, eicosanoids are signaling molecules made by oxygenation of twenty-carbon essential fatty acids, (EFAs). They exert complex control over many bodily systems, mainly in inflammation or immunity, and as messengers in the central nervous system. The networks of controls that depend upon eicosanoids are among the most complex in the human body.
Eicosanoids derive from either omega-3 (ω-3) or omega-6 (ω-6) EFAs. The ω-6 eicosanoids are generally pro-inflammatory; ω-3's are much less so. The amounts and balance of these fats in a person's diet will affect the body's eicosanoid-controlled functions, with effects on cardiovascular disease, triglycerides, blood pressure, and arthritis. Anti-inflammatory drugs such as aspirin and other NSAIDs act by downregulating eicosanoid synthesis.
There are four families of eicosanoids—the prostaglandins (PG), prostacyclins (PGI), the thromboxanes (TX) and the leukotrienes (LT). For each, there are two or three separate series, derived either from an ω-3 or ω-6 EFA. These series' different activities largely explain the health effects of ω-3 and ω-6 fats.[1] [2] [3] [4] [5]
Overall Description[edit]
When fed a fat-free diet, mammals will develop a deficiency disease—their skin becomes patchy, they are lethargic and cannot reproduce. Even small amounts of any of several polyunsaturated fats (PUFA—polyunsaturated fatty acids) will reverse this deficiency. Research shows there are two families of fats—the ω-3 and ω-6 fatty acids—that are necessary for bodily health; they are so-called the essential fatty acids (EFA).
These fats have several vital roles in the body. Most notably, they are the source of a bewilderingly[6] large family of lipid hormones—the eicosanoids—which control many different bodily functions. Fatty acid deficiency starves the body's ability to make eicosanoids; this explains most of the pathologies seen in the condition.[2] Eicosanoids orchestrate the movement of calcium, sodium and other ions into and out of cells. They control tissue dilation and contraction, the inhibition and promotion of clotting, regulation of digestive juices. They set the tempo of fertility, cell division and growth. But their most prominent functions—and the best studied—are their parts in inflammation.
The EFAs from vegetable sources (e.g. corn or canola oils) are short—with a chain eighteen carbons long, (short-chain EFA.) Foods from animals (and a few odd kelps) are the dietary source of long-chain EFA. The body makes eicosanoids from twenty-carbon long-chain EFA; they cannot be made from short-chain EFA directly. The body can—slowly—lengthen the short-chain EFA into long-chain EFA, but the process is inefficient. Long chain EFA are several-fold more potent in relieving EFA deficiency.[7]
Background on EFAs[edit]
- Any EFA can relieve the worst deficiency symptoms
- LC-EFA several times more effective than SC-EFA.
- ω-3 fats are also needed to form the brain. The long-chain ω-3 DHA is the most abundant fat in neural tissue, where it is the source of a related family of lipid hormones, the docosanoids.
Dietary sources[edit]
Paleo source[edit]
Series 2 as bad actors[edit]
in popular culture[edit]
Popularly, in the works of Barry Sears[8] and others,[9] the ω-6 eicosanoids are described as "bad" while the ω-3 are taken as "good".
Series 2 as necessary[edit]
Similarities in action[edit]
Humans must obtain both ω-3 and ω-6 EFA through our diet. Humans—and other vertebrates—cannnot synthesize them de novo. Both EFA types can support important physiological and developmental processes, can form eicosanoids, and can be metabolically elongated and desaturated to a variety of highly unsaturated fatty acids. The ω-6 acids are vigorously converted to potent ω-6 eicosanoids that exert intense agonist actions at eicosanoid receptors. But the ω-3 acids less vigorously form ω-3 eicosanoids that often produce less intense actions.[7]
Balance between ω-3 and ω-6[edit]
Both FA types travel the same biochemical pathways, from short-chain EFA, through conversion to long-chain EFA, incorporation into cell membranes, release in response to stimulation, conversion into eicosanoids, and actions at eicosanoid receptors. At each step, the ω-3 and ω-6 EFA are mutually inhibitory—they compete for the same cellular machinery. In particular, ω-3 fatty acids 'put the brakes on' the inflammatory ω-6 eicosanoids.[7] The two FA types are thus in dietary balance—a balance that ensures a balance between the ω-3 and ω-6 eicosanoids.
If the diet is poor in long-chain ω-3 (EPA and DHA) then the body corrects the balance, to some extent, by synthesizing more from vegetable sources (linoleic and linolenic acids).
This balancing can break down. Infants and young children—whose neural systems are growing rapidly—have greater needs for ω-3 EFA. Adult men do not have the same ability to synthesize long-chain EFA as well as women do, and the ability declines with age. Medical conditions such as diabetes interefere.[10] Trans fat inhibit Δ-6-desaturase—the enzyme that lengthens the EFA from 18 to 20 carbons. The resulting effect on eicosanoids is part of the mechanism by which trans fat promotes heart disease.[11] Deficiencies of biotin, vitamin E, protein, zinc, B12 and B6 all interfere with the action of Δ-6 desaturase and other enzymes involved in eicosanoid production.[12]
General list of actions[edit]
- PG strengthens the contractions of the heart.[13]
- Most investigated area is inflammation
- Initiation, resolution, chronic
Acute inflammation: [1]
- Marked by the accumulation of fluid edema and
- the migration of white blood cells (leukocytes, mainly neutrophils)
Leukotrienes in inflammation[edit]
Eicosanoids and the actions of leukotrienes[14]
- macrophages, neutrophils, platelets, and mast cells, all of which are involved in eicosanoid biosynthesis.[14]
- tumor necrosis factor and interleukin-1, can lead to arachidonic acid release and leukotriene formation in leukocytes
- Only white blood cells (specifically, myeloid cells) can make LTA. But they export it and other cells have the enzymes to convert it to LTB or LTC
- Active secretion of LTA4 has been shown in vitro in both mast cells and neutrophils
- Endothelial cells and platelets (= thrombocytes--control formation of clots) contain LTC4 synthase and can generate LTC4 from neutrophil-derived LTA4.
- In human bronchial or parenchymal strips, LTC4 and D4 exert a contractile potency 1000 times greater than either histamine or prostaglandin F2α
- Increased levels of LTC4 are found in the nasal lavage fluid of aspirin-intolerant asthmatic patients after challenge with aspirin
Site of synthesis steps, within the cell[edit]
- Influx of Ca++ through the cell membrane.
- EFA released from lipids in the cell membrane
- Two step process (AA -> HPETE -> LTA4)
- The cysteinyl leukotrienes
- finally undergo oxidative degradation by oxygen species released by activated phagocytes to form biologically inactive sulfoxides
- constituents of the slow-reacting substance of anaphylaxis.
- their capacity for inducing airway, gastrointestinal, and mesangial smooth-muscle contraction is perhaps the best characterized of the biological activities
- play a multifaceted role within the inflammatory process, inducing vasoconstriction, increasing vasopermeability, enhancing mucous secretion, and acting as immunomodulatory agents
Not circulating hormones, but on demand[edit]
Eicosanoids are fat-soluble; they can diffuse through lipid membranes. As such, they are not stored in vesicles. Instead, they are made rapidly, 'on demand', act at short distances, and are rapidly inactivated. Unlike the enodcrine hormones, which circulate throughout the body, eicosanoids act upon the cells that release them, or on their neighbors; (they are autocrine and paracrine hormones.)
Elongation and desaturation[edit]
Bio source of eicosanoids[edit]
- Perhaps some of the small detail should find a home in Eicosanoid biosynthesis.
- Must follow introduction to the ideas that
- The body makes the C20 it needs from C18.
- n-3 and n-6 in balance
Nomenclature[edit]
- See related detail at Essential Fatty Acid Interactions—Nomenclature
"Eicosanoid" (eicosa-, Greek for "twenty"; see icosahedron) is the collective term[15] for oxygenated derivatives of three different 20-carbon essential fatty acids:
- Eicosapentaenoic acid (EPA), an ω-3 fatty acid with 5 double bonds;
- Arachidonic acid (AA), an ω-6 fatty acid, with 4 double bonds;
- Dihomo-gamma-linolenic acid (DGLA), an ω-6, with 3 double bonds.
Derivation of names[edit]
- Holman proposed the ω nomenclature.[5]
- Who came up with the term eicosanoid?
- Prostaglandin from the prostate
- Thromboxane for the role in thrombosis
- Prostacyclin is a prostaglandin with an extra cycle.
- Leukotrienes affect leukocytes (how?) and have three conjugated double bonds.
- 'Arachidonic acid cascade' as the name.
Classic and Nonclassic Eicosanoids[edit]
Current usage limits the term eicosanoid to the leukotrienes and three types of prostanoids—prostaglandins prostacyclins, and thromboxanes. This is the definition used in this article. However, several other classes can technically be termed eicosanoid, including the hepoxilins, resolvins, isofurans, isoprostanes, lipoxins, epi-lipoxins, epoxyeicosatrienoic acids (EETs) and endocannabinoids. LTs and prostanoids are sometimes termed 'classic eicosanoids' [16][17][18] in contrast to the 'novel', 'eicosanoid-like' or 'nonclassic eicosanoids'.[19][20][21][22]
Blocks effects on the chain[edit]
Dietary blocks[edit]
trans fats, alcohol, protein deficiency?
Things that help[edit]
Antioxidants, lauric acid? Eating long chain
3/6 imbalance[edit]
Source of imbalance[edit]
Deficiencies as blocks[edit]
Enig: Deficiencies of biotin, vitamin E, protein, zinc, B12 and B6 all interfere with the action of delta-6 desaturase
Metabolic and health conditions as blocks[edit]
Pregnancy and lactation[edit]
Human breastmilk contains long chain EFA, but standard infant formula—at least until recently—has not. Blood concentrations of long chain EFA concentrations are markedly higher in breast-fed infants than the bottle-fed.[23]
Old age[edit]
Vegans and Vegetarians[edit]
Biosynthesis[edit]
Role in living things[edit]
Eicosanoids are found in most living things. In humans, eicosanoids are local hormones that are released by most cells, act on that same cell or nearby cells (i.e., they are autocrine and paracrine mediators), and then are rapidly inactivated. They are not stored within cells, but are synthesized as required. They derive from the fatty acids that make up the cell membrane and nuclear membrane.
Nomenclature for biosynthesis[edit]
Four character abbreviation[edit]
A particular eicosanoid is denoted by a four-character abbreviation, composed of:
- Its two letter abbreviation (above),[24]
- One A-B-C sequence-letter;[25] and
- A subscript, indicating the number of double bonds.
Examples are:
- The EPA-derived prostanoids have three double bonds, (e.g. PGG3, PGH3, PGI3, TXA3) while its leukotrienes have five, (LTB5).
- The AA-derived prostanoids have two double bonds, (e.g. PGG2, PGH2, PGI2, TXA2) while its leukotrienes have four, (LTB4).
Introduce COX and LO[edit]
Two families of enzymes catalyze fatty acid oxygenation to produce the eicosanoids:
- Cyclooxygenase, or COX, generates the prostanoids.
- Lipoxygenase, in several forms. 5-lipoxygenase (5-LO) generates the leukotrienes.
Overview of the pathway from EFA to (prostanoid or leukotriene) to inactivation[edit]
Biosynthesis initiation[edit]
Eicosanoid biosynthesis begins when cell is activated by mechanical trauma, cytokines, growth factors or other stimuli. (The stimulus may even be an eicosanoid from a neighboring cell; the pathways are complex.) Phospholipase is released at the cell wall and travels to the nuclear membrane. There, it frees 20-carbon essential fatty acids. This event appears to be the rate-determining step for eicosanoid formation.
Next, the free fatty acid is oxygenated along any of several pathways; see the Pathways table. The classical pathways add molecular oxygen (O2) via lipoxygenase or COX. Although the fatty acid is symmetric, the resulting eicosanoids are chiral; the oxidation proceeds with high stereospecificity.
Peroxidation and reactive oxygen species[edit]
The generation of eicosanoids is hazardous to the cell, particularly as it occurs close to the nucleus. There are elaborate mechanisms to prevent unwanted oxidation. COX, the lipoxygenases and the phospholipases are tightly controlled—there are at least eight proteins activated to coordinate generation of leukotrienes. Several of these exist in multiple isoforms.[4]
ROS can caused damage[edit]
Oxidation releases reactive oxygen species (ROS). Further, the initial products in eicosanoid generation are themselves highly reactive peroxides. E.g., LTA4 can form adducts with tissue DNA. Lipoxygenase can generate cellular damage; murine models implicate 15-lipoxygenase in the pathogenesis of atherosclerosis.[26][27]
Evolutionary purpose for peroxidation danger[edit]
What benefit does the cell realize from generating lipid hydroperoxides close-by its nucleus? PGs and LTs may signal or regulate DNA-transcription there; LTB4 is a ligand for PPARα,[2] (see diagram at PPAR). Perhaps eicosanoid signaling evolved from the detoxification of ROS. Several enzymes which are biosynthetic for eicosanoids belong to families whose functions are largely involved with cellular detoxification.
Prostanoid pathways[edit]
- Several drugs lower inflammation by blocking prostanoid synthesis; see detail at Cyclooxygenase, Aspirin and NSAID.
cox-1 and -2 invoked by different stimulii[edit]
competition for COX by n-3, -6[edit]
discovery of synthetic pathways[edit]
control of which prostanoid path after PGH[edit]
discussion of different stimuli and the resulting different products[edit]
control of removal, disactivation[edit]
sites of synthesis in the human body[edit]
use of inactive end-products to monitor short-lived prostanoids[edit]
Cyclooxygenase (COX) comes in at least three isoforms, COX-1, -2, -3.[28] COX-1 is responsible for basal prostaglandin synthesis, while COX-2 is important in inflammatory and "induced" settings.[2] COX-3 is active in nervous tissue.[3] COX catalyzes the conversion of the free essential fatty acids to prostanoids by a two-step process. First, two molecules of O2 are added as two peroxide linkages, and a 5-member carbon ring is forged near the middle of the fatty acid chain. This forms the short-lived, unstable intermediate Prostaglandin G (PGG). Next, one of the peroxide linkages sheds a single oxygen, forming PGH. (See diagrams and more detail of these steps at Cyclooxygenase).
Chemical characteristics of individual prostanoids[edit]
All three classes of prostanoids originate from PGH. All have distinctive rings in the center of the molecule. They differ in their structures. The PGH compounds (parents to all the rest) have a 5-carbon ring, bridged by two oxygens (a peroxide.) As the example in Structures of Selected Eicosanoids figure shows, the derived prostaglandins contain a single, unsaturated 5-carbon ring. In prostacyclins, this ring is conjoined to another oxygen-containing ring. In thromboxanes the ring becomes a 6-member ring with one oxygen. The leukotrienes do not have rings. (See more detail, including the enzymes involved, in diagrams at Prostanoid.)
Leukotriene pathways[edit]
cut much of the chemical detail[edit]
signals which initiate lk synthesis[edit]
n-3/n-6 competition[edit]
lipoxygenase inhibitors[edit]
sites in the body where lks are synthesized[edit]
overall control of lk production[edit]
The enzyme 5-lipoxygenase (5-LO) uses 5-lipoxygenase activating protein (FLAP) to convert arachidonic acid into 5-hydroperoxyeicosatetraenoic acid (5-HPETE), which spontaneously reduces to 5-hydroxyeicosatetraenoic acid (5-HETE). The enzyme 5-LO acts again on 5-HETE to convert it into leukotriene A4 (LTA4), which may be converted into LTB4 by the enzyme leukotriene A4 epoxide hydrolase. Eosinophils, mast cells, and alveolar macrophages use the enzyme leukotriene C4 synthase to conjugate glutathione with LTA4 to make LTC4, which is transported outside the cell, where a glutamic acid moiety is removed from it to make LTD4. The leukotriene LTD4 is then cleaved by dipeptidases to make LTE4. The leukotrienes LTC4, LTD4 and LTE4 all contain cysteine and are collectively known as the cysteinyl leukotrienes.
Function and pharmacology[edit]
| PGD2 | Promotion of sleep | TXA2 | Stimulation of platelet aggregation; vasoconstriction |
| PGE2 | Smooth muscle contraction; inducing pain, heat, fever; bronchoconstriction |
15d-PGJ2 | Adipocyte differentiation |
| PGF2α | Uterine contraction | LTB4 | Leukocyte chemotaxis |
| PGI2 | Inhibition of platelet aggregation; vasodilation; embryo implantation |
Cysteinyl-LTs | Anaphylaxis; bronchial smooth muscle contraction. |
| †Shown eicosanoids are AA-derived; EPA-derived generally have weaker activity | |||
Pharmo-kinetics[edit]
Eicosanoids have a short half-life, ranging from seconds to minutes.
Dietary effects on pharmacology[edit]
Dietary antioxidants inhibit the generation of some inflammatory eicosanoids, e.g. trans-resveratrol against thromboxane and some leukotrienes.[30]
Receptors[edit]
Most eicosanoid receptors are members of the G protein-coupled receptor superfamily; see the Receptors table or the article eicosanoid receptors.
Leukotrienes:
|
Prostanoids:
|
The ω-3 and ω-6 series[edit]
| “ | The reduction in AA-derived eicosanoids and the diminished activity of the alternative products generated from ω-3 fatty acids serve as the foundation for explaining some of the beneficial effects of greater ω-3 intake. | ” |
| — Kevin Fritsche, Fatty Acids as Modulators of the Immune Response[31] | ||
Arachidonic acid (AA; 20:4 ω-6) sits at the head of the 'arachidonic acid cascade'—more than twenty different eicosanoid-mediated signaling paths controlling a wide array of cellular functions, especially those regulating inflammation, immunity and the central nervous system.[3]
Overview of series-2 vs -3[edit]
Competing cascades[edit]
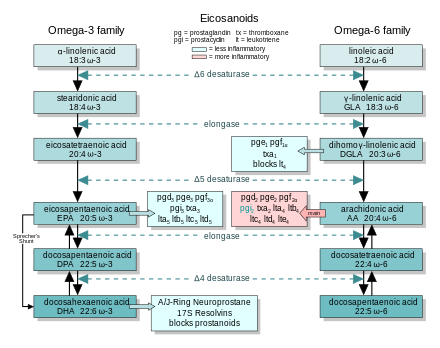
In the inflammatory response, two other groups of dietary essential fatty acids form cascades that parallel and compete with the arachidonic acid cascade. EPA (20:5 ω-3) provides the most important competing cascade. DGLA (20:3 ω-6) provides a third, less prominent cascade. These two parallel cascades soften the inflammatory effects of AA and its products. Low dietary intake of these less-inflammatory essential fatty acids, especially the ω-3s, has been linked to several inflammation-related diseases, and perhaps some mental illnesses.
Dietary recommendations on ω-3[edit]
The U.S. National Institutes of Health and the National Library of Medicine state that there is 'A' level evidence ('strong scientific evidence') that increased dietary ω-3 improves outcomes in hypertriglyceridemia, secondary cardiovascular disease prevention and hypertension. There is 'B' level evidence ('good scientific evidence') for increased dietary ω-3 in primary prevention of cardiovascular disease, rheumatoid arthritis and protection from ciclosporin toxicity in organ transplant patients. They also note more preliminary evidence showing that dietary ω-3 can ease symptoms in several psychiatric disorders.[32]
PUFA effects other than eicosanoids[edit]
Besides the influence on eicosanoids, dietary polyunsaturated fats modulate immune response through three other molecular mechanisms. They
(a) alter membrane composition and function, including the composition of lipid rafts;
(b) change cytokine biosynthesis and (c) directly activate gene transcription.[31] Of these, the action on eicosanoids is the best explored.
Mechanisms of ω-3 action[edit]
| “ | The arachidonic acid cascade is arguably the most elaborate signaling system neurobiologists have to deal with. | ” |
| — Daniele Piomelli, Arachidonic Acid[3] | ||
The eicosanoids from AA generally promote inflammation. Those from EPA and from GLA (via DGLA) are generally less inflammatory, or inactive, or even anti-inflammatory. The figure shows the ω-3 and -6 synthesis chains, along with the major eicosanoids from AA, EPA and DGLA.
Dietary ω-3 and GLA counter the inflammatory effects of AA's eicosanoids in three ways, along the eicosanoid pathways:
- Displacement—Dietary ω-3 decreases tissue concentrations of AA, so there is less to form ω-6 eicosanoids.
- Competitive inhibition—DGLA and EPA compete with AA for access to the cyclooxygenase and lipoxygenase enzymes. So the presence of DGLA and EPA in tissues lowers the output of AA's eicosanoids.
- Counteraction—Some DGLA and EPA derived eicosanoids counteract their AA derived counterparts.
Role in inflammation[edit]
Since antiquity, the cardinal signs of inflammation have been known as: calor (warmth), dolor (pain), tumor (swelling) and rubor (redness). The eicosanoids are involved with each of these signs.
| Medicine | Type | Medical condition or use |
|---|---|---|
| Alprostadil | PGI1 | Erectile dysfunction, maintaining a patent ductus arteriosus in the fetus |
| Beraprost | PGI1 analog | Pulmonary hypertension, avoiding reperfusion injury |
| Bimatoprost | PG analog | Glaucoma, ocular hypertension |
| Carboprost | PG analog | Labor induction, abortifacient in early pregnancy |
| Dinoprostone | PGE2 | Labor induction |
| Iloprost | PGI2 analog | Pulmonary arterial hypertension |
| Latanoprost | PG analog | Glaucoma, ocular hypertension |
| Misoprostol | PGE1 analog | Stomach ulcers, labor induction, abortifacient |
| Montelukast, Zafirlukast Pranlukast |
Cysteinal-LT receptor antagonists |
Asthma, seasonal allergies |
| Zileuton | 5-lipoxygenase inhibitor |
asthma |
| Travoprost | PG analog | Glaucoma, ocular hypertension |
| Treprostinil | PGI analog | Pulmonary hypertension |
| U46619 | Longer lived TX analog |
Research only |
| Zafirlukast | LT receptor antagonist |
Asthma |
Redness—An insect's sting will trigger the classic inflammatory response. Short acting vasoconstrictors—PGI2 and TXA2—are released quickly after the injury. The site may momentarily turn pale. Then TXA2 mediates the release of the vasodilators PGE2 and LTB4. The blood vessels engorge and the injury reddens.
Swelling—LTB4 makes the blood vessels more permeable. Plasma leaks out into the connective tissues, and they swell. The process also looses pro-inflammatory cytokines.
Pain—The cytokines increase COX-2 activity. This elevates levels of PGE2, sensitizing pain neurons.
Heat—PGE2 is a also potent pyretic agent. Aspirin and NSAIDS—drugs that block the COX pathways and stop prostanoid synthesis—limit fever or the heat of localized inflammation.
Action of prostanoids[edit]
- Main articles: Prostaglandin, Prostacyclin and Thromboxane
Prostanoids mediate local symptoms of inflammation: vasoconstriction or vasodilation, coagulation, pain and fever. COX-2 is responsible for pain and inflammation, while COX-1 is responsible for platelet clotting actions. Prostanoids play pivotal functions inflammation, platelet aggregation, and vasoconstriction/relaxation.
Signals in nucleus[edit]
Prostanoids activate the PPARγ members of the steroid/thyroid family of nuclear hormone receptors, directly influencing gene transcription.[33]
Pharma role of COX[edit]
Inhibition of cyclooxygenase, specifically the inducible COX-2 isoform, is the hallmark of NSAIDs (non-steroidal anti-inflammatory drugs), such as aspirin.
Action of leukotrienes[edit]
- Main article: Leukotriene
Leukotrienes play an important role in inflammation. There is a neuroendocrine role for LTC4 in luteinizing hormone secretion.[34] LTB4 causes adhesion and chemotaxis of leukocytes and stimulates aggregation, enzyme release, and generation of superoxide in neutrophils.[35] Blocking leukotriene receptors can play a role in the management of inflammatory diseases such as asthma (by the drugs montelukast and zafirlukast), psoriasis, and rheumatoid arthritis.
slow reacting substance of anaphylaxis[edit]
The slow reacting substance of anaphylaxis comprises the cysteinyl leukotrienes. These have a clear role in pathophysiological conditions such as asthma, allergic rhinitis and other nasal allergies, and have been implicated in atherosclerosis and inflammatory gastrointestinal diseases.[36] They are potent bronchoconstrictors, increase vascular permeability in postcapillary venules, and stimulate mucus secretion. They are released from the lung tissue of asthmatic subjects exposed to specific allergens and play a pathophysiological role in immediate hypersensitivity reactions.[35] Along with PGD, they function in effector cell trafficking, antigen presentation, immune cell activation, matrix deposition, and fibrosis.[37]
History[edit]
In 1930, gynecologist Raphael Kurzrok and pharmacologist Charles Leib characterized prostaglandin as a component of semen. Between 1929 and 1932, Burr and Burr showed that restricting fat from animal's diets led to a deficiency disease, and first described the essential fatty acids.[38] In 1935, von Euler identified prostaglandin. In 1964, Bergström and Samuelsson linked these observations when they showed that the "classical" eicosanoids were derived from arachidonic acid, which had earlier been considered to be one of the essential fatty acids.[39] In 1971, Vane showed that aspirin and similar drugs inhibit prostaglandin synthesis.[40] Von Euler received the Nobel Prize in medicine in 1970, which Samuelsson, Vane, and Bergström also received in 1982. E. J. Corey received it in chemistry in 1990 largely for his synthesis of prostaglandins.
References[edit]
- ^ a b DeCaterina, R and Basta, G (June, 2001). "n-3 Fatty acids and the inflammatory response – biological background" (PDF). European Heart Journal Supplements. 3, Suppl D: D42–D49. doi:10.1016/S1520-765X(01)90118-X. Retrieved 2006-02-10.
{{cite journal}}: Check date values in:|date=(help)CS1 maint: multiple names: authors list (link) - ^ a b c d Funk, Colin D. (30 November 2001). "Prostaglandins and Leukotrienes: Advances in Eicosanoid Biology". Science. 294 (5548): 1871–1875. doi:10.1126/science.294.5548.1871. PMID 11729303. Retrieved 2007-01-08.
- ^ a b c d Piomelli, Daniele (2000). "Arachidonic Acid". Neuropsychopharmacology: The Fifth Generation of Progress. Retrieved 2006-03-03.
- ^ a b Soberman, Roy J. and Christmas, Peter (2003). "The organization and consequences of eicosanoid signaling". J. Clin. Invest. 111 (8): 1107–1113. doi:10.1172/JCI200318338. Retrieved 2007-01-05.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ a b Holman RT (1998). "The slow discovery of the importance of omega 3 essential fatty acids in human health" (pdf). J. Nutr. 128 (2 Suppl): 427S–433S. doi:10.1093/jn/128.2.427S. PMID 9478042. Retrieved 2007-11-13.
- ^ William S. Harris. "Role of Omega-3 Fatty Acids in Cardiovascular Disease Prevention". Baylor University. Retrieved 2007-12-18.
- ^ a b c Lands WE (1992). "Biochemistry and physiology of n-3 fatty acids" (PDF). FASEB J. 6 (8): 2530–6. doi:10.1096/fasebj.6.8.1592205. PMID 1592205.
{{cite journal}}: CS1 maint: unflagged free DOI (link) - ^ Sears, Barry (2002). The Omega Rx Zone : The Miracle of the New High-Dose Fish Oil. New York: Regan Books, HarperCollins. ISBN 0-06-098919-X. Retrieved 2007-11-21.
- ^ Cavanagh, A. "Overcome depression with natural therapies". Retrieved 2007-11-21.
- ^ Brenner RR, Peluffo RO, Mercuri O, Restelli MA (1968). "Effect of arachidonic acid in the alloxan-diabetic rat". Am. J. Physiol. 215 (1): 63–70. doi:10.1152/ajplegacy.1968.215.1.63. PMID 5659344.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Hu FB, Manson JE, Willett WC (2001). "Types of dietary fat and risk of coronary heart disease: a critical review". Journal of the American College of Nutrition. 20 (1): 5–19. doi:10.1080/07315724.2001.10719008. PMID 11293467.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Horrobin DF (1983). "The regulation of prostaglandin biosynthesis by the manipulation of essential fatty acid metabolism". Reviews in Pure & Applied Pharmacological Sciences. 4 (4): 339–83. PMID 6400557.
- ^ Schrör K, Hohlfeld T (1992). "Inotropic actions of eicosanoids". Basic Res. Cardiol. 87 (1): 2–11. doi:10.1007/BF00795384. PMID 1314558.
- ^ a b Henderson WR (1994). "The role of leukotrienes in inflammation". Ann. Intern. Med. 121 (9): 684–97. doi:10.7326/0003-4819-121-9-199411010-00010. PMID 7944079. Retrieved 2007-11-17.
- ^ Beare-Rogers (2001). "IUPAC Lexicon of Lipid Nutrition" (PDF). Retrieved June 1, 2006.
- ^ Van Dyke TE, Serhan CN (2003). "Resolution of inflammation: a new paradigm for the pathogenesis of periodontal diseases". J. Dent. Res. 82 (2): 82–90. doi:10.1177/154405910308200202. PMID 12562878.
- ^ Serhan CN, Gotlinger K, Hong S, Arita M (2004). "Resolvins, docosatrienes, and neuroprotectins, novel omega-3-derived mediators, and their aspirin-triggered endogenous epimers: an overview of their protective roles in catabasis". Prostaglandins Other Lipid Mediat. 73 (3–4): 155–72. doi:10.1016/j.prostaglandins.2004.03.005. PMID 15290791.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Anderle P, Farmer P, Berger A, Roberts MA (2004). "Nutrigenomic approach to understanding the mechanisms by which dietary long-chain fatty acids induce gene signals and control mechanisms involved in carcinogenesis". Nutrition (Burbank, Los Angeles County, Calif.). 20 (1): 103–8. doi:10.1016/j.nut.2003.09.018. PMID 14698023.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Evans AR, Junger H, Southall MD; et al. (2000). "Isoprostanes, novel eicosanoids that produce nociception and sensitize rat sensory neurons". J. Pharmacol. Exp. Ther. 293 (3): 912–20. PMID 10869392.
{{cite journal}}: Explicit use of et al. in:|author=(help)CS1 maint: multiple names: authors list (link) - ^ O'Brien WF, Krammer J, O'Leary TD, Mastrogiannis DS (1993). "The effect of acetaminophen on prostacyclin production in pregnant women". Am. J. Obstet. Gynecol. 168 (4): 1164–9. doi:10.1016/0002-9378(93)90362-m. PMID 8475962.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Behrendt H, Kasche A, Ebner von Eschenbach C, Risse U, Huss-Marp J, Ring J (2001). "Secretion of proinflammatory eicosanoid-like substances precedes allergen release from pollen grains in the initiation of allergic sensitization". Int. Arch. Allergy Immunol. 124 (1–3): 121–5. doi:10.1159/000053688. PMID 11306946.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Sarau HM, Foley JJ, Schmidt DB; et al. (1999). "In vitro and in vivo pharmacological characterization of SB 201993, an eicosanoid-like LTB4 receptor antagonist with anti-inflammatory activity". Prostaglandins Leukot. Essent. Fatty Acids. 61 (1): 55–64. doi:10.1054/plef.1999.0074. PMID 10477044.
{{cite journal}}: Explicit use of et al. in:|author=(help)CS1 maint: multiple names: authors list (link) - ^ Horrobin DF (1993). "Fatty acid metabolism in health and disease: the role of delta-6-desaturase" (pdf). Am. J. Clin. Nutr. 57 (5 Suppl): 732S–736S, discussion 736S–737S. doi:10.1093/ajcn/57.5.732S. PMID 8386433. Retrieved 2007-11-17.
- ^ Prostacyclin—PGI—was previously classified as prostaglandin and retains its old identifier.
- ^ Eicosanoids with different letters have placement of double-bonds and different functional groups attached to the molecular skeleton. Letters indicate roughly the order the eicosanoids were first described in the literature. For diagrams for PG [A–H] see Cyberlipid Center. "Prostanoids". Retrieved 2007-02-05.
- ^ Cyrus, Tillmann (June 1999). "Disruption of the 12/15-lipoxygenase gene diminishes atherosclerosis in apo E–deficient mice". J Clin Invest. 103 (11): 1597–1604n. doi:10.1172/JCI5897. PMC 408369. PMID 10359569.
- ^ Schewe T. (2002 Mar-Apr). "15-lipoxygenase-1: a prooxidant enzyme". Biol Chem. 383 (3–4): 312–314. doi:10.1007/BF01940809. PMID 3428. Retrieved 2007-01-09.
{{cite journal}}: Check date values in:|date=(help) - ^ Warner, Timothy D. and Mitchell, Jane A. (October 8, 2002). "Cyclooxygenase-3 (COX-3): Filling in the gaps toward a COX continuum?". PNAS. 99 (21): 13371–13373. doi:10.1073/pnas.222543099. PMC 129677. PMID 12374850.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ University of Kansas Medical Center (2004). "Eicosanoids and Inflammation" (PDF). Retrieved 2007-01-05.
- ^ Pace-Asciak CR, Hahn S, Diamandis EP, Soleas G, Goldberg DM. (1995 Mar 31). "The red wine phenolics trans-resveratrol and quercetin block human platelet aggregation and eicosanoid synthesis: implications for protection against coronary heart disease". Clin Chim Acta. 235 (2): 207–19. doi:10.1016/0009-8981(95)06045-1. PMID 7554275.
{{cite journal}}: Check date values in:|date=(help)CS1 maint: multiple names: authors list (link) - ^ a b Fritsche, Kevin (August 2006). "Fatty Acids as Modulators of the Immune Response". Annual Review of Nutrition. 26: 45–73. doi:10.1146/annurev.nutr.25.050304.092610. PMID 16848700. Retrieved 2007-01-11.
- ^ National Institute of Health (August 1, 2005). "Omega-3 fatty acids, fish oil, alpha-linolenic acid". Retrieved March 26, 2006.
- ^ Bos C, Richel D, Ritsema T, Peppelenbosch M, Versteeg H (2004). "Prostanoids and prostanoid receptors in signal transduction". Int J Biochem Cell Biol. 36 (7): 1187–205. doi:10.1016/j.biocel.2003.08.006. PMID 15109566.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Samuelsson, SE Dahlen, JA Lindgren, CA Rouzer, and CN Serhan (1987). "Leukotrienes and lipoxins: structures, biosynthesis, and biological effects". Science. 237 (4819): 1171–1176. doi:10.1126/science.2820055. PMID 2820055. Retrieved 2007-01-22.
{{cite journal}}: Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - ^ a b Samuelsson B (May 1983). "Leukotrienes: mediators of immediate hypersensitivity reactions and inflammation". Science. 220 (4597): 568–575. doi:10.1126/science.6301011. PMID 6301011.
{{cite journal}}: CS1 maint: date and year (link) - ^ Capra V (2004). "Molecular and functional aspects of human cysteinyl leukotriene receptors". Pharmacol Res. 50 (1): 1–11. doi:10.1016/j.phrs.2003.12.012. PMID 15082024.
- ^ Boyce J (2005). "Eicosanoid mediators of mast cells: receptors, regulation of synthesis, and pathobiologic implications". Chem Immunol Allergy. Chemical Immunology and Allergy. 87: 59–79. doi:10.1159/000087571. ISBN 3-8055-7948-9. PMID 16107763.
- ^ Burr, G.O. and Burr, M.M. (1930). "On the nature and role of the fatty acids essential in nutrition" (PDF). J. Biol. Chem. 86 (587): 587–621. doi:10.1016/S0021-9258(20)78929-5. Retrieved 2007-01-17.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Bergström, S., Danielsson, H. and Samuelsson, B. (1964). "The enzymatic formation of prostaglandin E2 from arachidonic acid". Biochim. Biophys. Acta. 90 (207): 207–210. doi:10.1016/0304-4165(64)90145-x. PMID 14201168.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Vane, J. R. (23 June 1971). "Inhibition of prostaglandin synthesis as a mechanism of action for aspirin-like drugs". Nature New Biol. 231 (25): 232–5. doi:10.1038/newbio231232a0. PMID 5284360.
External links[edit]
- Eicosanoids at the U.S. National Library of Medicine Medical Subject Headings (MeSH)