User:Cygispex/sandbox2
| Bdelloid rotifers | |
|---|---|

| |
| SEM showing morphological variation of bdelloid rotifers and their jaws | |
| Scientific classification | |
| Kingdom: | |
| Superphylum: | |
| Phylum: | |
| Class: | Bdelloidea
|
Bdelloidea /ˈdɛlɔɪdiə/ (Greek βδελλα, bdella, "leech-like") is a class of rotifers found in freshwater habitats all over the world. There are over 450 described species of bdelloid rotifers (or 'bdelloids'), distinguished from each other mainly on the basis of morphology [1]. The main characteristics that distinguish bdelloids from related groups of rotifers are exclusively parthenogenetic reproduction and the ability to survive in dry, harsh environments by entering a state of desiccation-induced dormancy (anhydrobiosis) at any life stage[2]. Bdelloid rotifers are microscopic organisms, typically between 150 and 700 µm in length[2]. They are slightly too small to be seen with the naked eye, but appear as small white dots through even a weak hand lens.
Taxonomy[edit]
Evolutionary Relationships[edit]
The phylum Rotifera traditionally included three classes: Bdelloidea, Monogononota and Seisonidea[3]. Prior to 1990, phylogenetic studies based on morphology seemed to indicate that the sister group to bdelloid rotifers was Monogononta, with seisonid rotifers as an early-diverging outgroup.[4]

Modern molecular phylogenetic studies demonstrate that this classic understanding of 'Rotifera' is incomplete (paraphyletic), because it omits a fourth clade of closely related organisms: the Acanthocephala, or thorny-headed worms[7] . Originally classified as a separate phylum, molecular and morphological evidence accumulated between 1994 and 2014 to indicate that Acanthocephala forms a monophyletic group with Bdelloidea, Monogononta and Seisonidea [5][8]. To accommodate this finding, some authors extend the term 'Rotifera' to include the highly modified, parasitic 'acanthocephalan rotifers' alongside bdelloid, monogonont and seisonid rotifers [9]. Others refer to the grouping of the four taxa as Syndermata, a term derived from their shared syncytial epidermis[8].
The position of Bdelloidea within Syndermata (or Rotifera) is not entirely clear. Alternative possible phylogenetic relationships within the clade are illustrated by the accompanying cladograms. As of 2014, the "most comprehensive phylogenomic analysis of syndermatan relationships" to date was based on transcriptome data from all four groups [5], and provided "strong support" for the hypothesis illustrated in the bottom left of the figure, in which Seisonidea and Acanthocephala are sister taxa. The study further indicated that the sister group to this taxon is Bdelloidea, whereas Monogononta is the outgroup to all three. This would mean that the closest living relatives of bdelloid rotifers are not monogonont rotifers, as previously believed, but seisonid rotifers and acanthocephalans, despite their highly modified morphology.
Classification and Identification[edit]
The class Bdelloidea consists of three orders: Philodinavida, Philodinida and Adinetida. These orders are divided into four families, 19 genera and about 450 species.[10] Since these organisms are asexual the definition of species is expanded outwith the common, but not only, definition of "organisms capable of creating fertile offspring", therefore the confirmation of each seperate species is completed through a mixture of morphological and molecular evidence.
Bdelloids can only be identified by eye whilst they are alive because many of the characteristics that are significant are to do with feeding and crawling, genetic identification of bdelloids is possible on dead individuals however. Once preserved, the individuals contract into "blobs" which restricts analysis.[11] There are currently three morphological identification methodologies, two of which are considered dated by Bartôs (1951)[12] and Donner (1965).[10] The third method is a diagnostic key developed in 1995 by Shiel.[11]
Morphology[edit]
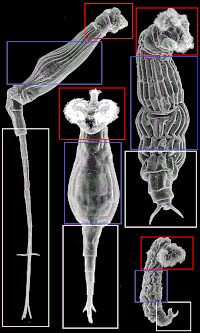
Fully grown individuals of some species may be as long as 1600 µm (Rotaria neptunia), or as short as 77 µm (Habrotrocha minuta) [10].
There are three main regions of the body of bdelloids: head, trunk and foot. The image on the right depicts each area to show how body parts can be very different although they are named the same depending on the species involved. Bdelloids typically have a well-developed corona, divided into two parts, on a retractable head.
Some identifiable features of the bdelloids include :
- Well-developed foot glands [11]
- A mouth opening with a long oesophagus [11]
- Strong teeth (labelled by a tooth index) [11]
- Many cilia [11]
- Species-specific upper lip shape [11]
- Order-specific corona type [2]
- Philodinida consist of two ciliated discs
- Adinetida consist of a ventral ciliated field
- Philodinavida have a small corona
The bdelloid digestive and reproductive systems can be found within the trunk sections of their bodies, with the stomach being the most visible of the organs. In certain genera, (Habrotrocha, Otostephanos and Scepanotrocha) the bdelloid can actually be identified by the appearance of distinct spherical pellets within the stomach, which will be released as faeces. These pellets are a distinguishing characteristic since all the other genera release faeces as loose material.[2]
Most bdelloids retract the foot whilst they eat, but there are four genera that lack a foot - Adineta, Bradyscela, Henoceros and Philodinavus. This affects not only how they feed but also how they crawl; for instance Adineta and Bradyscela slide whereas the other genera loop.[2]
Ecology[edit]
The behaviour of bdelloids can be split into four categories: feeding, locomotion, reproduction and stress-induced behaviours.
Habitat[edit]
Bdelloid rotifers are found in nearly all fresh water habitats of any size[13]. They are especially common in temporarily moist habitats such as soils and thin films of water on the surface of lichens, mosses and other plants. A few species occur in brackish water, and even fewer in marine habitats[10]. Some bdelloid rotifer species live commensally on the exterior of larger animals, such as the water louse Asellus aquaticus[10].
Feeding[edit]
The specifics feeding behaviour of bdelloids is varied although most follow a generalised theme: They use rings of cilia in the corona organ to create currents of water which blow food through the mouth to the mastrax organ which has been adapted specifically for grinding food and has a 'trophi' feature which is similar to jaws.[14] Food includes suspended bacteria, algae and detritus amongst others as well.
Locomotion[edit]
There appears to be three main methods of movement: free swimming, inch-worming along a substrate or sessility. Inch-worming (or crawling) involves taking alternate steps with the head and tail, as do certain leeches, which gives the group their name (Greek βδελλα or bdella, meaning leech). This video demonstrates how bdelloids move in three different situations: locomotion and feeding of bdelloid rotifers.
Reproduction[edit]
Bdelloids are of interest in the study of the evolution of sex because a male has never been observed, and females reproduce exclusively by parthenogenesis, a form of asexual reproduction where embryos grow and develop without the need for fertilization; this is part of the apomixis process.[15] Each individual has paired gonads. Despite having been asexual for millions of years, they have diversified into more than 450 species and are fairly similar to other sexually reproducing rotifer species.
Stress induced behaviour[edit]
Bdelloids are able to survive environmental stresses by entering a state of dormancy known as anhydrobiosis which enables the organism to rapidly dehydrate and thus resist desiccation. While preparing for this dormant state many metabolic processes are adjusted to equate for the change in state; e.g. the production of protective chemicals.[16] The bdelloid can remain in this state, which is known as a 'tun' until the return of a sufficient amount of water, at which point they will rehydrate and become active within hours. Hatching of the young will only occur when conditions are at their most favourable.[17] These forms of dormancy are also known as cryptobiosis or quiescence. Bdelloids have been known to survive in this state for up to 9 years whilst waiting for favourable conditions to return. [17]
When these creatures recover from hibernation, it has been shown that they undergo a possibly unique genetic process where HTG has occured resulting in a significant proportion of the bdelloid genome, upto 10%, having been obtained through HTG with containing with bacteria, fungi and plants.[18] The how, when and why HTG occur with bdelloids is under much debate at present; particularly with regards to possible connections between the foreign genes and the dessication process as well as possible connections to bdelloids ancient asexuality.
Bdelloid rotifers are extraordinarily resistant to damage from ionizing radiation due to the same DNA-preserving adaptations used to survive dormancy. [19]
Images[edit]
Pictures of bdelloids to assist in visualising the anatomy.
-
Lateral view of a bdelloid.
-
Frontal view of a bdelloid's corona.
-
Lateral view of a bdelloid.
-
Lateral view of a bdelloid.
-
Lateral view of a Bdelloid Rotifer in algae-rich water
See also[edit]
References[edit]
- ^ Segers, Hendrick (2007). Annotated checklist of the rotifers (Phylum Rotifera), with notes on nomenclature, taxonomy and distribution (PDF). Auckland: Magnolia Press. ISBN 978-1-86977-129-4.
- ^ a b c d e Ricci C (2000). "Key to the identification of the genera of bdelloid rotifers". Hydrobiologia. 418: 73–80. doi:10.1023/A:1003840216827.
- ^ King, Charles E.; Ricci, Claudia; Schonfeld, Justin; Serra, Manuel (September 2005). "Evolutionary Dynamics of 'the' Bdelloid and Monogonont Rotifer Life-history Patterns". Hydrobiologia. 546 (1): 55–70. doi:10.1007/s10750-005-4102-9.
- ^ Lee Wallace, Robert; Colburn, Rebecca Arlene (December 1989). "Phylogenetic relationships within phylum Rotifera: orders and genus Notholca". Hydrobiologia. 186–187 (1): 311–318. doi:10.1007/BF00048926.
- ^ a b c Wey-Fabrizius, Alexandra R.; Herlyn, Holger; Rieger, Benjamin; Rosenkranz, David; Witek, Alexander; Welch, David B. Mark; Ebersberger, Ingo; Hankeln, Thomas (10 February 2014). "Transcriptome Data Reveal Syndermatan Relationships and Suggest the Evolution of Endoparasitism in Acanthocephala via an Epizoic Stage". PLOS ONE. 9 (2): e88618. doi:10.1371/journal.pone.0088618. PMC 3919803. PMID 24520404.
- ^ Lasek-Nesselquist, Erica (23 August 2012). "A Mitogenomic Re-Evaluation of the Bdelloid Phylogeny and Relationships among the Syndermata". PLOS ONE. 7 (8): e43554. doi:10.1371/journal.pone.0043554. PMC 3426538. PMID 22927990.
- ^ Mark Welch, David B. (2001). Hydrobiologia. 446/447: 315–322. doi:10.1023/A:1017502923286.
{{cite journal}}: Missing or empty|title=(help) - ^ a b Ahlrichs, W. H. (21 March 1997). "Epidermal ultrastructure of Seison nebaliae and Seison annulatus , and a comparison of epidermal structures within the Gnathifera". Zoomorphology. 117 (1): 41–48. doi:10.1007/s004350050028.
- ^ Nielsen, Claus (2012). Animal evolution : interrelationships of the living phyla (3rd ed.). Oxford: Oxford University Press. ISBN 978-0199606030.
- ^ a b c d e Donner, Josef (1965). Ordnung Bdelloidea. Akademie-Verlag. p. 297. ISBN 9789031908851.
- ^ a b c d e f g Shiel, R J (1995). A guide to identification of rotifers, cladocerans and copepods from Australian inland waters. Australia: Co-operative Research Centre for Freshwater Ecology. ISBN 0646224107.
- ^ Bartôs B (1954). "The Czechoslovak Rotatoria of the order Bdelloidea". Memoires de la Societe Zoologique Tchecoslovaque de Prague. 15: 241–500.
- ^ Ricci, Claudia N. (April 1987). "Ecology of bdelloids: how to be successful". Hydrobiologia. 147 (1): 117–127. doi:10.1007/BF00025734.
- ^ Klusemann J (1990). "The hard parts (trophi) of the rotifer mastax do contain chitin: evidence from studies on Brachionus plicatilis". Histochem. Cell Biol. 3: 277–283.
- ^ Milius S. "Bdelloids: No sex for over 40 million years". TheFreeLibrary. ScienceNews. Retrieved March 2014.
{{cite web}}: Check date values in:|accessdate=(help) - ^ Crowe J (1971). "Anhydrobiosis: an unsolved problem". American Naturalist. 105 (946): 563–573. doi:10.1086/282745. JSTOR 2459752.
- ^ a b Guidetti R (2002). "Long-term anhydrobiotic survival in semi-terrestrial micrometazoans". Journal of Zoology. 257 (2): 181–187. doi:10.1017/S095283690200078X.
- ^ Boschetti C (20011). "Foreign genes and novel hydrophilic protein genes participate in the desiccation response of the bdelloid rotifer Adineta ricciae". The Journal of Experimental Biology. 214 (Pt 1): 59–68. doi:10.1242/jeb.050328. PMID 21147969.
{{cite journal}}: Check date values in:|year=(help) - ^ Gladyshev E (2008). "Extreme resistance of bdelloid rotifers to ionizing radiation". Proceedings of the National Academy of Sciences. 105 (13): 5139–5144. doi:10.1073/pnas.0800966105. PMID 18362355.






