User:Chem540f09grp3/Sandbox
(From original wiki page) In some cases, additional rate enhancement is seen for the lighter isotope, possibly due to the quantum mechanical tunneling. This is typically only observed for reactions involving bonds to hydrogen. (End of original wiki page)
Tunneling occurs when a molecule penetrates through a potential energy barrier rather than over it. [1][2] Although not allowed by the laws of classical mechanics, particles can pass through classically forbidden regions of space in quantum mechanics based on wave-particle duality.[3]

Analysis of tunneling can be made using Bell’s modification of the Arrhenius equation which includes the addition of a tunneling factor, Q:
where A is the Arrhenius parameter, E is the barrier height and
where
and
Examination of the β term shows exponential dependency on the mass of the particle. As a result, tunneling is much more likely for a lighter particle such as hydrogen. Simply doubling the mass of a tunneling proton by replacing it with its deuterium isotope drastically reduces the rate of such reactions. As a result, very large kinetic isotope effects are observed that can not be accounted for by differences in zero point energies.

In addition, the β term depends linearly with barrier width, 2a. As with mass, tunneling is greatest for small barrier widths. Optimal tunneling distances of protons between donor and acceptor atom is 0.4 Å.[5]
Temperature Dependance in Tunneling[edit]
Tunneling is a quantum mechanical effect tied to the laws of wave mechanics, not kinetics. Therefore, tunneling tends to become more important at low temperatures, where even the smallest kinetic energy barriers may not be overcome but can be tunneled through.[1]
Peter S. Zuev et. al. reported rate constants for the ring expansion of 1-methylcyclobutylfluorocarbene to be 4.0 x 10-6/s in nitrogen and 4.0 x 10-5/s in argon at 8 kelvin. They calculated that at 8 kelvin, the reaction would proceed via a single quantum state of the reactant so that the reported rate constant is temperature independent and the tunneling contribution to the rate was 152 orders of magnitude greater than the contribution of passage over the transition state energy barrier.[6]
So despite the fact that conventional chemical reactions tend to slow down dramatically as the temperature is lowered, tunneling reactions rarely change at all. Particles that tunnel through an activation barrier are a direct result of the fact that the wave function of an intermediate species, reactant or product is not confined to the energy well of a particular trough along the energy surface of a reaction but can “leak out” into the next energy minimum. In light of this, tunneling should be temperature independent.[1] [7]
Noboru Fujisaki et. al. reported the gas phase H/D kinetic isotope effect for the hydrogen abstraction from some n-alkanes and cycloalkanes by hydrogen atoms over the temperature range 363-463 K. The data they obtained was characterized by small preexponential factor ratios AH/AD ranging from 0.43 to 0.54 and large activation energy differences from 9.0 to 9.7 kJ/mol. Basing their arguments on transition state theory, the small A factor ratios associated with the large activation energy differences (usually about 4.5 kJ/mol for C-H(D) bonds) provided strong evidence for tunneling. For the purpose of this discussion, what is important to note is that the A factor ratio for the various paraffins they used was approximately constant throughout the temperature range.[8]
The observed fact that tunneling isn't entirely temperature independent can be explained by the fact that not all molecules of a certain species occupy their vibrational ground state at varying temperatures. Adding thermal energy to a potential energy well could cause higher vibrational levels other than the ground state to become populated. For a conventional kinetically driven reaction, this excitation would only have a small influence on the rate. However, for a tunneling reaction, the difference between the zero point energy and the first vibrational energy level could be huge. The tunneling correction term Q is linearly dependent on barrier width and this width is significantly diminished as the number vibrational modes on the Morse potential increase. The decrease of the barrier width can have such a huge impact on the tunneling rate that even a small population of excited vibrational states would dominate this process.[1] [7]
Criteria for KIE tunneling[edit]
To determine if tunneling is involved in KIE of a reaction with H or D, there are a few criteria:
1.Δ(EaH-EaD) > Δ(ZPEH-ZPED) (Ea=activation energy; ZPE=zero point energy)
2.Reaction still proceeds at lower temperatures.
3.The Arrhenius pre-exponential factors AD/AH is not equal to 1.
4.There is a large negative entropy of activation.
5.The geometries of the reactants and products are usually very similar.[1]
Also for reactions where isotopes include H, D and T, a criterion of tunneling is the Swain-Schaad relations which compare the rate constants(k) of the reactions when H,D or T are the protons:
kH/kT=(kD/kT)X and kH/kT=(kH/kD)Y
Experimental values of X exceeding 3.26 and Y lower than 1.44 are evidence of a certain amount of contribution from tunneling. [5]
Examples[edit]
(From original Wiki page) In organic reactions, this proton tunneling effect has been observed in such reactions as the deprotonation and iodination of nitropropane with hindered pyridine base[9] with a reported KIE of 25 at 25 °C:
and in a 1,5-sigmatropic hydrogen shift[10] although it is observed that it is difficult to extrapolate experimental values obtained at elevated temperatures to lower temperatures:[11][12]
There has long been speculation that high efficiency of enzyme catalysis in proton or hydride ion transfer reactions could be due partly to the quantum mechanical tunneling effect. Environment at the active site of an enzyme positions the donor and acceptor atom close to the optimal tunneling distance, where the amino acid side chains can "force" the donor and acceptor atom closer together by electrostatic and noncovalent interactions. It is also possible that the enzyme and its unusual hydrophobic environment inside a reaction site provides tunneling-promoting vibration. [13]
Studies on ketosteroid isomerase have provided experimental evidence that the enzyme actually enhances the coupled motion/hydrogen tunneling by comparing primary and secondary kinetic isotope effects of the reaction under enzyme catalyzed and non-enzyme catalyzed conditions.[14]
There are many examples of proton tunneling in enzyme catalyzed reactions that were discovered by KIE. A well studied example is methylamine dehydrogenase, where large primary KIEs of 5-55 have been observed for the proton transfer step. [15]

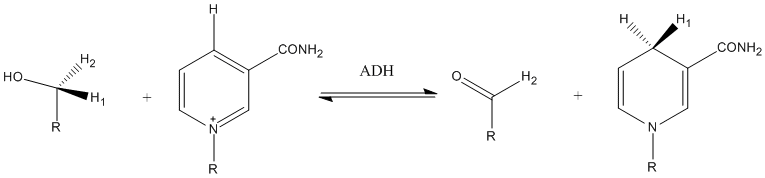
Another example of tunneling contribution to proton transfer in enzymatic reactions is the reaction carried out by alcohol dehydrogenase. Competitive KIEs for the hydrogen transfer step at 25 °C resulted in 3.6 and 10.2 for primary and secondary KIEs, respectively. [16]
References[edit]
- ^ a b c d e f E.V. Anslyn, D.A. Dougherty (2006). Modern Physical Organic Chemistry. University Science Books. pp. 435–437. ISBN 1-891389-31-9. Cite error: The named reference "anslyn" was defined multiple times with different content (see the help page).
- ^ M.Razauy (2003). Quantum Theory of Tunneling. World Scientific Publishing Company. ISBN 9-812380-19-1.
- ^ R.J. Silbey, R.A. alberty, M.G. Bawendi (2005). Physical Chemistry. John Wiley and Sons, Inc. pp. 326–338. ISBN 0-471215-04-X.
{{cite book}}: CS1 maint: multiple names: authors list (link) - ^ Borgis, D., Hynes, J.T. (1993). Chemical Physics. 170: 315–346. doi:10.1016/0301-0104(93)85117-Q.
{{cite journal}}: Missing or empty|title=(help)CS1 maint: multiple names: authors list (link) - ^ a b Lev I. Krishtalik (2000). Biochimica et Biopysica Acta. 1458: 6–27. doi:10.1016/S0005-2728(00)00057-8.
{{cite journal}}: Missing or empty|title=(help) - ^ Peter S. Zuev; et al. (2003). Science. 299: 867. doi:10.1126/science.1079294.
{{cite journal}}: Explicit use of et al. in:|author=(help); Missing or empty|title=(help) - ^ a b Peter Atkins, Julio de Paula (2006). Atkins' Physical Chemistry. Oxford University Press. pp. 286–288, 816–818. ISBN 9780198700722.
- ^ Noboru Fujisaki; et al. (1987). J. Phys. Chem. 91: 1602. doi:0022-3654/87/2091-1606$01.50/0.
{{cite journal}}: Check|doi=value (help); Explicit use of et al. in:|author=(help); Missing or empty|title=(help) - ^ Edward Sheldon Lewis and Lance Funderburk (1967). "Rates and isotope effects in the proton transfers from 2-nitropropane to pyridine bases". J. Am. Chem. Soc. 89 (10): 2322–2327. doi:10.1021/ja00986a013.
- ^ Michael J. S. Dewar, Eamonn F. Healy, and James M. Ruiz (1988). "Mechanism of the 1,5-sigmatropic hydrogen shift in 1,3-pentadiene". J. Am. Chem. Soc. 110 (8): 2666–2667. doi:10.1021/ja00216a060.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ William von E. Doering and Xin Zhao (2006). "Effect on Kinetics by Deuterium in the 1,5-Hydrogen Shift of a Cisoid-Locked 1,3(Z)-Pentadiene, 2-Methyl-10-methylenebicyclo[4.4.0]dec-1-ene: Evidence for Tunneling?". J. Am. Chem. Soc. 128 (28): 9080–9085. doi:10.1021/ja057377v.
- ^ In this study the KIE is measured by sensitive proton NMR. The extrapolated KIE at 25 °C is 16.6 but the margin of error is high
- ^ Amnon Kohen; Judith P Klinman (1999). Chem. Biol. 6: R191-198. doi:10.1016/S1074-5521(99)80058-1.
{{cite journal}}: Missing or empty|title=(help) - ^ Thomas C. Wilde; Grzegorz Blotny; Ralph M. Pollack (2008). J. Am. Chem. Soc. . 130: 6577–6585. doi:10.1021/ja0732330.
{{cite journal}}: Missing or empty|title=(help) - ^ Donald G. Truhlar; Jiali Gao; Cristobal Alhambra; Mireia Garcia-Viloca; José Corchado; Maria Luz Sánchez; Jordi Villà (2002). Acc. Chem. Res. 35: 341–349. doi:10.1021/ar0100226.
{{cite journal}}: Missing or empty|title=(help) - ^ Amnon Kohen; Judtih P. Klinman (1998). Acc. Chem. Res. 31: 397–404. doi:10.1021/ar9701225.
{{cite journal}}: Missing or empty|title=(help)






