User:CFCF/sandbox/LC
{{Infobox medical condition/simplified/sandbox |Name = Lung cancer |Image = LungCACXR.PNG |Caption = A chest X-ray showing a tumor in the lung (marked by arrow) |field = Oncology |DiseasesDB = 7616 |ICD10 = C33-C34 |ICD9 = 162 |ICDO = |OMIM =211980 |MedlinePlus = 007194 |eMedicineSubj = med |eMedicineTopic = 1333 |eMedicine_mult = med/1336 emerg/335 radio/807 radio/405 radio/406 |MeshID = D002283 }} Lung cancer, also known as carcinoma of the lung or pulmonary carcinoma, is a malignant lung tumor characterized by uncontrolled cell growth in tissues of the lung. If left untreated, this growth can spread beyond the lung by process of metastasis into nearby tissue or other parts of the body. Most cancers that start in the lung, known as primary lung cancers, are carcinomas that derive from epithelial cells. The main primary types are small-cell lung carcinoma (SCLC) and non-small-cell lung carcinoma (NSCLC). The most common symptoms are coughing (including coughing up blood), weight loss, shortness of breath, and chest pains.[1]
The vast majority (80–90%) of cases of lung cancer are due to long-term exposure to tobacco smoke.[1][2] About 10–15% of cases occur in people who have never smoked.[3] These cases are often caused by a combination of genetic factors[4] and exposure to radon gas,[4] asbestos,[5] or other forms of air pollution,[4] including second-hand smoke.[6][7] Lung cancer may be seen on chest radiographs and computed tomography (CT) scans. The diagnosis is confirmed by biopsy[8] which is usually performed by bronchoscopy or CT-guidance.
Treatment and long-term outcomes depend on the type of cancer, the stage (degree of spread), and the person's overall health, measured by performance status. Common treatments include surgery, chemotherapy, and radiotherapy. NSCLC is sometimes treated with surgery, whereas SCLC usually responds better to chemotherapy and radiotherapy.[9] Overall, 16.8% of people in the United States diagnosed with lung cancer survive five years after the diagnosis,[10] while outcomes on average are worse in the developing world. Worldwide, lung cancer is the most common cause of cancer-related death in men and women, and was responsible for 1.56 million deaths annually, as of 2012.[11]
Signs and symptoms[edit]
Signs and symptoms which may suggest lung cancer include:[1]
- Respiratory symptoms: coughing, coughing up blood, wheezing or shortness of breath
- Systemic symptoms: weight loss, fever, clubbing of the fingernails, or fatigue
- Symptoms due to the cancer mass pressing on adjacent structures: chest pain, bone pain, superior vena cava obstruction, difficulty swallowing
If the cancer grows in the airways, it may obstruct airflow, causing breathing difficulties. The obstruction can lead to accumulation of secretions behind the blockage, and predispose to pneumonia.[1]
Depending on the type of tumor, paraneoplastic phenomena—symptoms not due to the local presence of cancer—may initially attract attention to the disease.[12] In lung cancer, these phenomena may include Lambert–Eaton myasthenic syndrome (muscle weakness due to autoantibodies), hypercalcemia, or syndrome of inappropriate antidiuretic hormone (SIADH, abnormally concentrated urine and diluted blood). Tumors in the top of the lung, known as Pancoast tumors, may invade the local part of the sympathetic nervous system, leading to Horner's syndrome (dropping of the eyelid and a small pupil on that side), as well as damage to the brachial plexus.[1]
Many of the symptoms of lung cancer (poor appetite, weight loss, fever, fatigue) are not specific.[8] In many people, the cancer has already spread beyond the original site by the time they have symptoms and seek medical attention.[13] Symptoms that suggest the presence of metastatic disease include weight loss, bone pain and neurological symptoms (headaches, fainting, convulsions, or limb weakness).[1] Common sites of spread include the brain, bone, adrenal glands, opposite lung, liver, pericardium, and kidneys.[13] About 10% of people with lung cancer do not have symptoms at diagnosis; these cancers are incidentally found on routine chest radiography.[14]
Causes[edit]

Cancer develops following genetic damage to DNA and epigenetic changes. These changes affect the normal functions of the cell, including cell proliferation, programmed cell death (apoptosis) and DNA repair. As more damage accumulates, the risk of cancer increases.[15]
Smoking[edit]
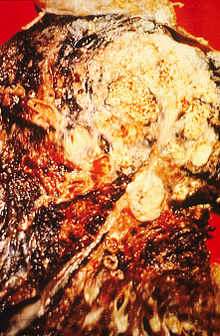
Smoking, particularly of cigarettes, is by far the main contributor to lung cancer.[16] Cigarette smoke contains at least 73 known carcinogens,[17] including benzo[a]pyrene,[18] NNK, 1,3-butadiene and a radioactive isotope of polonium, polonium-210.[17] Across the developed world, 90% of lung cancer deaths in men during the year 2000 were attributed to smoking (70% for women).[19] Smoking accounts for 80–90% of lung cancer cases.[1]
Passive smoking—the inhalation of smoke from another's smoking—is a cause of lung cancer in nonsmokers. A passive smoker can be defined as someone living or working with a smoker. Studies from the US,[20][21][22] Europe[23] and the UK[24] have consistently shown a significantly increased risk among those exposed to passive smoke.[25] Those who live with someone who smokes have a 20–30% increase in risk while those who work in an environment with secondhand smoke have a 16–19% increase in risk.[26] Investigations of sidestream smoke suggest it is more dangerous than direct smoke.[27] Passive smoking causes about 3,400 deaths from lung cancer each year in the USA.[22]
Marijuana smoke contains many of the same carcinogens as those in tobacco smoke.[28] However the effect of smoking cannabis on lung cancer risk is not clear.[29][30] A 2013 review did not find an increased risk from light to moderate use.[31] A 2014 review found that smoking cannabis doubled the risk of lung cancer.[32]
Radon gas[edit]
Radon is a colourless and odorless gas generated by the breakdown of radioactive radium, which in turn is the decay product of uranium, found in the Earth's crust. The radiation decay products ionize genetic material, causing mutations that sometimes turn cancerous. Radon is the second-most common cause of lung cancer in the USA,[33] causing about 21,000 deaths each year.[34] The risk increases 8–16% for every 100 Bq/m³ increase in the radon concentration.[35] Radon gas levels vary by locality and the composition of the underlying soil and rocks. About one in 15 homes in the US has radon levels above the recommended guideline of 4 picocuries per liter (pCi/l) (148 Bq/m³).[36]
Asbestos[edit]
Asbestos can cause a variety of lung diseases, including lung cancer. Tobacco smoking and asbestos have a synergistic effect on the formation of lung cancer.[5] In smokers who work with asbestos, the risk of lung cancer is increased 45-fold compared to the general population.[37] Asbestos can also cause cancer of the pleura, called mesothelioma (which is different from lung cancer).[38]
Air pollution[edit]
Outdoor air pollution has a small effect on increasing the risk of lung cancer.[4] Fine particulates (PM2.5) and sulfate aerosols, which may be released in traffic exhaust fumes, are associated with slightly increased risk.[4][39] For nitrogen dioxide, an incremental increase of 10 parts per billion increases the risk of lung cancer by 14%.[40] Outdoor air pollution is estimated to account for 1–2% of lung cancers.[4]
Tentative evidence supports an increased risk of lung cancer from indoor air pollution related to the burning of wood, charcoal, dung or crop residue for cooking and heating.[41] Women who are exposed to indoor coal smoke have about twice the risk and a number of the by-products of burning biomass are known or suspected carcinogens.[42] This risk affects about 2.4 billion people globally,[41] and is believed to account for 1.5% of lung cancer deaths.[42]
Genetics[edit]
About 8% of lung cancer is due to inherited factors.[43] In relatives of people with lung cancer, the risk is increased 2.4 times. This is likely due to a combination of genes.[44] Polymorphisms on chromosomes 5, 6 and 15 are known to affect the risk of lung cancer.[45]
Other causes[edit]
Numerous other substances, occupations, and environmental exposures have been linked to lung cancer. The International Agency for Research on Cancer (IARC) states there is "sufficient evidence" to show the following are carcinogenic in the lungs:[46]
- Some metals (aluminum production, cadmium and cadmium compounds, chromium(VI) compounds, beryllium and beryllium compounds, iron and steel founding, nickel compounds, arsenic and inorganic arsenic compounds, underground hematite mining)
- Some products of combustion (incomplete combustion, coal (indoor emissions from household coal burning), coal gasification, coal-tar pitch, coke production, soot, diesel engine exhaust)
- Ionizing radiation (X-radiation, gamma radiation, plutonium)
- Some toxic gases (methyl ether (technical grade), Bis-(chloromethyl) ether, sulfur mustard, MOPP (vincristine-prednisone-nitrogen mustard-procarbazine mixture), fumes from painting)
- Rubber production and crystalline silica dust
Pathogenesis[edit]
Similar to many other cancers, lung cancer is initiated by activation of oncogenes or inactivation of tumor suppressor genes.[47] Carcinogens cause mutations in these genes which induce the development of cancer.[48]
Mutations in the K-ras proto-oncogene are responsible for 10–30% of lung adenocarcinomas.[49][50] About 4% of non-small-cell lung carcinomas involve an EML4-ALK tyrosine kinase fusion gene.[51]
Epigenetic changes—such as alteration of DNA methylation, histone tail modification, or microRNA regulation—may lead to inactivation of tumor suppressor genes.[52]
The epidermal growth factor receptor (EGFR) regulates cell proliferation, apoptosis, angiogenesis, and tumor invasion.[49] Mutations and amplification of EGFR are common in non-small-cell lung carcinoma and provide the basis for treatment with EGFR-inhibitors. Her2/neu is affected less frequently.[49] Other genes that are often mutated or amplified are c-MET, NKX2-1, LKB1, PIK3CA, and BRAF.[49]
The cell lines of origin are not fully understood.[1] The mechanism may involve abnormal activation of stem cells. In the proximal airways, stem cells that express keratin 5 are more likely to be affected, typically leading to squamous-cell lung carcinoma. In the middle airways, implicated stem cells include club cells and neuroepithelial cells that express club cell secretory protein. Small-cell lung carcinoma may be derived from these cell lines[53] or neuroendocrine cells,[1] and may express CD44.[53]
Metastasis of lung cancer requires transition from epithelial to mesenchymal cell type. This may occur through activation of signaling pathways such as Akt/GSK3Beta, MEK-ERK, Fas, and Par6.[54]
Diagnosis[edit]

Performing a chest radiograph is one of the first investigative steps if a person reports symptoms that may suggest lung cancer. This may reveal an obvious mass, widening of the mediastinum (suggestive of spread to lymph nodes there), atelectasis (collapse), consolidation (pneumonia) or pleural effusion.[2] CT imaging is typically used to provide more information about the type and extent of disease. Bronchoscopy or CT-guided biopsy is often used to sample the tumor for histopathology.[14]
Lung cancer often appears as a solitary pulmonary nodule on a chest radiograph. However, the differential diagnosis is wide. Many other diseases can also give this appearance, including tuberculosis, fungal infections, metastatic cancer or organizing pneumonia. Less common causes of a solitary pulmonary nodule include hamartomas, bronchogenic cysts, adenomas, arteriovenous malformation, pulmonary sequestration, rheumatoid nodules, granulomatosis with polyangiitis, or lymphoma.[55] Lung cancer can also be an incidental finding, as a solitary pulmonary nodule on a chest radiograph or CT scan done for an unrelated reason.[56] The definitive diagnosis of lung cancer is based on histological examination of the suspicious tissue in the context of the clinical and radiological features.[1]
Clinical practice guidelines recommend frequencies for pulmonary nodule surveillance.[57] CT imaging should not be used for longer or more frequently than indicated as extended surveillance exposes people to increased radiation.[57]
Classification[edit]
| Histological type | Incidence per 100,000 per year |
|---|---|
| All types | 66.9 |
| Adenocarcinoma | 22.1 |
| Squamous-cell carcinoma | 14.4 |
| Small-cell carcinoma | 9.8 |
Lung cancers are classified according to histological type.[8] This classification is important for determining management and predicting outcomes of the disease. Lung cancers are carcinomas—malignancies that arise from epithelial cells. Lung carcinomas are categorized by the size and appearance of the malignant cells seen by a histopathologist under a microscope. For therapeutic purposes, two broad classes are distinguished: non-small-cell lung carcinoma and small-cell lung carcinoma.[58]
Non-small-cell lung carcinoma[edit]

The three main subtypes of NSCLC are adenocarcinoma, squamous-cell carcinoma and large-cell carcinoma.[1]
Nearly 40% of lung cancers are adenocarcinoma, which usually originates in peripheral lung tissue.[8] Although most cases of adenocarcinoma are associated with smoking, adenocarcinoma is also the most common form of lung cancer among people who have smoked fewer than 100 cigarettes in their lifetimes ("never-smokers").[1][59] A subtype of adenocarcinoma, the bronchioloalveolar carcinoma, is more common in female never-smokers, and may have a better long-term survival.[60]
Squamous-cell carcinoma accounts for about 30% of lung cancers. They typically occur close to large airways. A hollow cavity and associated cell death are commonly found at the centre of the tumour.[8] About 9% of lung cancers are large-cell carcinoma. These are so named because the cancer cells are large, with excess cytoplasm, large nuclei and conspicuous nucleoli.[8]
Small-cell lung carcinoma[edit]

In small-cell lung carcinoma (SCLC), the cells contain dense neurosecretory granules (vesicles containing neuroendocrine hormones), which give this tumor an endocrine/paraneoplastic syndrome association.[61] Most cases arise in the larger airways (primary and secondary bronchi).[14] These cancers grow quickly and spread early in the course of the disease. Sixty to seventy percent have metastatic disease at presentation. This type of lung cancer is strongly associated with smoking.[1]
Others[edit]
Four main histological subtypes are recognised, although some cancers may contain a combination of different subtypes.[58] Rare subtypes include glandular tumors, carcinoid tumors, and undifferentiated carcinomas.[1]
Metastasis[edit]
| Histological type | Immunostain |
|---|---|
| Squamous-cell carcinoma | CK5/6 positive CK7 negative |
| CK7 positive TTF-1 positive | |
| Large-cell carcinoma | TTF-1 negative |
| Small-cell carcinoma | TTF-1 positive CD56 positive Chromogranin positive Synaptophysin positive |
The lung is a common place for the spread of tumours from other parts of the body. Secondary cancers are classified by the site of origin; e.g., breast cancer that has spread to the lung is called metastatic breast cancer. Metastases often have a characteristic round appearance on chest radiograph.[62]
Primary lung cancers themselves most commonly metastasize to the brain, bones, liver and adrenal glands.[8] Immunostaining of a biopsy is often helpful to determine the original source.[63]
Staging[edit]
Lung cancer staging is an assessment of the degree of spread of the cancer from its original source. It is one of the factors affecting the prognosis and potential treatment of lung cancer.[1]
The initial evaluation of non-small-cell lung carcinoma (NSCLC) staging uses the TNM classification. This is based on the size of the primary tumor, lymph node involvement, and distant metastasis.[1]
|
|
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Using the TNM descriptors, a group is assigned, ranging from occult cancer, through stages 0, IA (one-A), IB, IIA, IIB, IIIA, IIIB and IV (four). This stage group assists with the choice of treatment and estimation of prognosis.[65]
| TNM | Stage group |
|---|---|
| T1a–T1b N0 M0 | IA |
| T2a N0 M0 | IB |
| T1a–T2a N1 M0 | IIA |
| T2b N0 M0 | |
| T2b N1 M0 | IIB |
| T3 N0 M0 | |
| T1a–T3 N2 M0 | IIIA |
| T3 N1 M0 | |
| T4 N0–N1 M0 | |
| N3 M0 | IIIB |
| T4 N2 M0 | |
| M1 | IV |
Small-cell lung carcinoma (SCLC) has traditionally been classified as "limited stage" (confined to one half of the chest and within the scope of a single tolerable radiotherapy field) or "extensive stage" (more widespread disease).[1] However, the TNM classification and grouping are useful in estimating prognosis.[65]
For both NSCLC and SCLC, the two general types of staging evaluations are clinical staging and surgical staging. Clinical staging is performed prior to definitive surgery. It is based on the results of imaging studies (such as CT scans and PET scans) and biopsy results. Surgical staging is evaluated either during or after the operation, and is based on the combined results of surgical and clinical findings, including surgical sampling of thoracic lymph nodes.[8]
- Diagrams of main features of staging
-
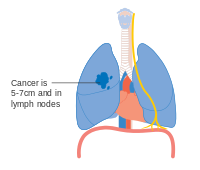
Stage IA and IB lung cancer
-
Stage IIA lung cancer
-
Stage IIB lung cancer
-
One option for stage IIB lung cancer, with T2b; but if tumour is within 2 cm of the carina, this is stage 3
-
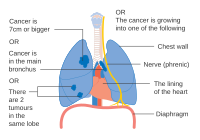
Stage IIIA lung cancer
-
Stage IIIA lung cancer, if there is one feature from the list on each side
-
Stage IIIA lung cancer
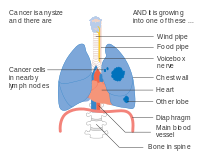
-
Stage IIIB lung cancer
-
Stage IIIB lung cancer
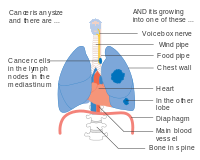
-
Stage IV lung cancer
Prevention[edit]
Smoking prevention and smoking cessation are effective ways of preventing the development of lung cancer.[66]
Smoking ban[edit]
While in most countries industrial and domestic carcinogens have been identified and banned, tobacco smoking is still widespread. Eliminating tobacco smoking is a primary goal in the prevention of lung cancer, and smoking cessation is an important preventive tool in this process.[67]
Policy interventions to decrease passive smoking in public areas such as restaurants and workplaces have become more common in many Western countries.[68] Bhutan has had a complete smoking ban since 2005[69] while India introduced a ban on smoking in public in October 2008.[70] The World Health Organization has called for governments to institute a total ban on tobacco advertising to prevent young people from taking up smoking. They assess that such bans have reduced tobacco consumption by 16% where instituted.[71]
Screening[edit]
Cancer screening uses medical tests to detect disease in large groups of people who have no symptoms.[72] For individuals with high risk of developing lung cancer, computed tomography (CT) screening can detect cancer and give a person options to respond to it in a way that prolongs life.[57] This form of screening reduces the chance of death from lung cancer by an absolute amount of 0.3% (relative amount of 20%).[73][74] High risk people are those age 55-74 who have smoked equivalent amount of a pack of cigarettes daily for 30 years including time within the past 15 years.[57]
CT screening is associated with a high rate of falsely positive tests which may result in unneeded treatment.[75] For each true positive scan there are about 19 falsely positives scans.[76] Other concerns include radiation exposure[75] and the cost of testing along with follow up.[57] Research has not found two other available tests—sputum cytology or chest radiograph (CXR) screening tests—to have any benefit.[77]
The U.S. Preventative Services Task Force (USPSTF) recommends yearly screening using low-dose computed tomography in those who have a total smoking history of 30 pack-years and are between 55 to 80 years old until a person has not been smoking for more than 15 years.[78] Screening should not be done in those with other health problems that would make treatment of lung cancer if found not an option.[78] The English National Health Service was in 2014 re-examining the evidence for screening.[79]
Other prevention strategies[edit]
The long-term use of supplemental vitamin A,[80][81] vitamin C,[80] vitamin D[82] or vitamin E[80] does not reduce the risk of lung cancer. Some studies suggest that people who eat diets with a higher proportion of vegetables and fruit tend to have a lower risk,[22][83] but this may be due to confounding—with the lower risk actually due to the association of a high fruit/vegetables diet with less smoking. More rigorous studies have not demonstrated a clear association between diet and lung cancer risk.[83]
Management[edit]
Treatment for lung cancer depends on the cancer's specific cell type, how far it has spread, and the person's performance status. Common treatments include palliative care,[84] surgery, chemotherapy, and radiation therapy.[1] Targeted therapy of lung cancer is growing in importance for advanced lung cancer.
Surgery[edit]

If investigations confirm NSCLC, the stage is assessed to determine whether the disease is localized and amenable to surgery or if it has spread to the point where it cannot be cured surgically. CT scan and positron emission tomography are used for this determination.[1] If mediastinal lymph node involvement is suspected, mediastinoscopy may be used to sample the nodes and assist staging.[85] Blood tests and pulmonary function testing are used to assess whether a person is well enough for surgery.[14] If pulmonary function tests reveal poor respiratory reserve, surgery may not be a possibility.[1]
In most cases of early-stage NSCLC, removal of a lobe of lung (lobectomy) is the surgical treatment of choice. In people who are unfit for a full lobectomy, a smaller sublobar excision (wedge resection) may be performed. However, wedge resection has a higher risk of recurrence than lobectomy.[86] Radioactive iodine brachytherapy at the margins of wedge excision may reduce the risk of recurrence.[87] Rarely, removal of a whole lung (pneumonectomy) is performed.[86] Video-assisted thoracoscopic surgery (VATS) and VATS lobectomy use a minimally invasive approach to lung cancer surgery.[88] VATS lobectomy is equally effective compared to conventional open lobectomy, with less postoperative illness.[89]
In SCLC, chemotherapy and/or radiotherapy is typically used.[90] However the role of surgery in SCLC is being reconsidered. Surgery might improve outcomes when added to chemotherapy and radiation in early stage SCLC.[91]
Radiotherapy[edit]
Radiotherapy is often given together with chemotherapy, and may be used with curative intent in people with NSCLC who are not eligible for surgery. This form of high-intensity radiotherapy is called radical radiotherapy.[92] A refinement of this technique is continuous hyperfractionated accelerated radiotherapy (CHART), in which a high dose of radiotherapy is given in a short time period.[93] Postoperative thoracic radiotherapy generally should not be used after curative intent surgery for NSCLC.[94] Some people with mediastinal N2 lymph node involvement might benefit from post-operative radiotherapy.[95]
For potentially curable SCLC cases, chest radiotherapy is often recommended in addition to chemotherapy.[8]

If cancer growth blocks a short section of bronchus, brachytherapy (localized radiotherapy) may be given directly inside the airway to open the passage. Compared to external beam radiotherapy, brachytherapy allows a reduction in treatment time and reduced radiation exposure to healthcare staff.[96] Evidence for brachytherapy, however, is less than that for external beam radiotherapy.[97]
Prophylactic cranial irradiation (PCI) is a type of radiotherapy to the brain, used to reduce the risk of metastasis. PCI is most useful in SCLC. In limited-stage disease, PCI increases three-year survival from 15% to 20%; in extensive disease, one-year survival increases from 13% to 27%.[98]
Recent improvements in targeting and imaging have led to the development of stereotactic radiation in the treatment of early-stage lung cancer. In this form of radiotherapy, high doses are delivered over a number of sessions using stereotactic targeting techniques. Its use is primarily in patients who are not surgical candidates due to medical comorbidities.[99]
For both NSCLC and SCLC patients, smaller doses of radiation to the chest may be used for symptom control (palliative radiotherapy).[100]
Chemotherapy[edit]
The chemotherapy regimen depends on the tumor type.[8] Small-cell lung carcinoma (SCLC), even relatively early stage disease, is treated primarily with chemotherapy and radiation.[101] In SCLC, cisplatin and etoposide are most commonly used.[102] Combinations with carboplatin, gemcitabine, paclitaxel, vinorelbine, topotecan, and irinotecan are also used.[103][104] In advanced non-small cell lung carcinoma (NSCLC), chemotherapy improves survival and is used as first-line treatment, provided the person is well enough for the treatment.[105] Typically, two drugs are used, of which one is often platinum-based (either cisplatin or carboplatin). Other commonly used drugs are gemcitabine, paclitaxel, docetaxel,[106][107] pemetrexed,[108] etoposide or vinorelbine.[107]
Adjuvant chemotherapy refers to the use of chemotherapy after apparently curative surgery to improve the outcome. In NSCLC, samples are taken of nearby lymph nodes during surgery to assist staging. If stage II or III disease is confirmed, adjuvant chemotherapy improves survival by 5% at five years.[109][110] The combination of vinorelbine and cisplatin is more effective than older regimens.[110] Adjuvant chemotherapy for people with stage IB cancer is controversial, as clinical trials have not clearly demonstrated a survival benefit.[111][112] Chemotherapy before surgery in NSCLC that can be removed surgically also appears to improve outcomes.[113]
Chemotherapy may be combined with palliative care in the treatment of the NSCLC. In advanced cases, appropriate chemotherapy improves average survival over supportive care alone, as well as improving quality of life.[114] With adequate physical fitness maintaining chemotherapy during lung cancer palliation offers 1.5 to 3 months of prolongation of survival, symptomatic relief, and an improvement in quality of life, with better results seen with modern agents.[115][116] The NSCLC Meta-Analyses Collaborative Group recommends if the recipient wants and can tolerate treatment, then chemotherapy should be considered in advanced NSCLC.[105][117]
Targeted therapy[edit]
Several drugs that target molecular pathways in lung cancer are available, especially for the treatment of advanced disease. Erlotinib, gefitinib and afatinib inhibit tyrosine kinase at the epidermal growth factor receptor. Denosumab is a monoclonal antibody directed against receptor activator of nuclear factor kappa-B ligand. It may be useful in the treatment of bone metastases.[118]
Palliative care[edit]
Palliative care when added to usual cancer care benefits people even when they are still receiving chemotherapy.[119] These approaches allow additional discussion of treatment options and provide opportunities to arrive at well-considered decisions.[120][121] Palliative care may avoid unhelpful but expensive care not only at the end of life, but also throughout the course of the illness. For individuals who have more advanced disease, hospice care may also be appropriate.[14][121]
Prognosis[edit]
| Clinical stage | Five-year survival (%) | |
|---|---|---|
| Non-small-cell lung carcinoma | Small-cell lung carcinoma | |
| IA | 50 | 38 |
| IB | 47 | 21 |
| IIA | 36 | 38 |
| IIB | 26 | 18 |
| IIIA | 19 | 13 |
| IIIB | 7 | 9 |
| IV | 2 | 1 |
Of all people with lung cancer in the US, 16.8% survive for at least five years after diagnosis.[10][122] In England, between 2005 and 2009, overall five-year survival for lung cancer was less than 10%.[123] Outcomes are generally worse in the developing world.[124] Stage is often advanced at the time of diagnosis. At presentation, 30–40% of cases of NSCLC are stage IV, and 60% of SCLC are stage IV.[8] Survival for lung cancer falls as the stage at diagnosis becomes more advanced: the English data suggest that around 70% of patients survive at least a year when diagnosed at the earliest stage, but this falls to just 14% for those diagnosed with the most advanced disease.[125]
Prognostic factors in NSCLC include presence or absence of pulmonary symptoms, tumor size, cell type (histology), degree of spread (stage) and metastases to multiple lymph nodes, and vascular invasion. For people with inoperable disease, outcomes are worse in those with poor performance status and weight loss of more than 10%.[126] Prognostic factors in small cell lung cancer include performance status, gender, stage of disease, and involvement of the central nervous system or liver at the time of diagnosis.[127]
For NSCLC, the best prognosis is achieved with complete surgical resection of stage IA disease, with up to 70% five-year survival.[128] For SCLC, the overall five-year survival is about 5%.[1] People with extensive-stage SCLC have an average five-year survival rate of less than 1%. The average survival time for limited-stage disease is 20 months, with a five-year survival rate of 20%.[2]
According to data provided by the National Cancer Institute, the median age at diagnosis of lung cancer in the United States is 70 years,[129] and the median age at death is 72 years.[130] In the US, people with medical insurance are more likely to have a better outcome.[131]
Epidemiology[edit]

no data ≤ 5 5-10 10-15 15-20 20-25 25-30 | 30-35 35-40 40-45 45-50 50-55 ≥ 55 |
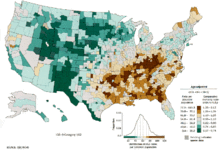

Worldwide, lung cancer is the most common cancer among men in terms of both incidence and mortality, and among women has the third highest incidence, and is second after breast cancer in mortality. In 2012, there were 1.82 million new cases globally, and 1.56 million deaths due to lung cancer, representing 19.4% of all deaths from cancer.[11] The highest rates are in North America, Europe and East Asia, with over a third of new cases in 2012 in China. Rates in Africa and South Asia are much lower.[133]
The population segment most likely to develop lung cancer is people aged over 50 who have a history of smoking. In contrast to the mortality rate in men, which began declining more than 20 years ago, women's lung cancer mortality rates have been rising over the last decades, and are just recently beginning to stabilize.[134] In the USA, the lifetime risk of developing lung cancer is 8% in men and 6% in women.[1]
For every 3–4 million cigarettes smoked, one lung cancer death occurs.[1][135] The influence of "Big Tobacco" plays a significant role in the smoking culture.[136] Young nonsmokers who see tobacco advertisements are more likely to take up smoking.[137] The role of passive smoking is increasingly being recognized as a risk factor for lung cancer,[25] leading to policy interventions to decrease undesired exposure of nonsmokers to others' tobacco smoke.[138] Emissions from automobiles, factories, and power plants also pose potential risks.[4]
Eastern Europe has the highest lung cancer mortality among men, while northern Europe and the US have the highest mortality among women. In the United States, black men and women have a higher incidence.[139] Lung cancer rates are currently lower in developing countries.[140] With increased smoking in developing countries, the rates are expected to increase in the next few years, notably in China[141] and India.[142]
Lung cancer is the second most common cancer in the UK (around 43,500 people were diagnosed with the disease in 2011), and it is the most common cause of cancer death (around 35,400 people died in 2012).[143]
From the 1960s, the rates of lung adenocarcinoma started to rise relative to other types of lung cancer. This is partly due to the introduction of filter cigarettes. The use of filters removes larger particles from tobacco smoke, thus reducing deposition in larger airways. However, the smoker has to inhale more deeply to receive the same amount of nicotine, increasing particle deposition in small airways where adenocarcinoma tends to arise.[144] The incidence of lung adenocarcinoma continues to rise.[145]
History[edit]
Lung cancer was uncommon before the advent of cigarette smoking; it was not even recognized as a distinct disease until 1761.[146] Different aspects of lung cancer were described further in 1810.[147] Malignant lung tumors made up only 1% of all cancers seen at autopsy in 1878, but had risen to 10–15% by the early 1900s.[148] Case reports in the medical literature numbered only 374 worldwide in 1912,[149] but a review of autopsies showed the incidence of lung cancer had increased from 0.3% in 1852 to 5.66% in 1952.[150] In Germany in 1929, physician Fritz Lickint recognized the link between smoking and lung cancer,[148] which led to an aggressive antismoking campaign.[151] The British Doctors' Study, published in the 1950s, was the first solid epidemiological evidence of the link between lung cancer and smoking.[152] As a result, in 1964 the Surgeon General of the United States recommended smokers should stop smoking.[153]
The connection with radon gas was first recognized among miners in the Ore Mountains near Schneeberg, Saxony. Silver has been mined there since 1470, and these mines are rich in uranium, with its accompanying radium and radon gas.[154] Miners developed a disproportionate amount of lung disease, eventually recognized as lung cancer in the 1870s.[155] Despite this discovery, mining continued into the 1950s, due to the USSR's demand for uranium.[154] Radon was confirmed as a cause of lung cancer in the 1960s.[156]
The first successful pneumonectomy for lung cancer was performed in 1933.[157] Palliative radiotherapy has been used since the 1940s.[158] Radical radiotherapy, initially used in the 1950s, was an attempt to use larger radiation doses in patients with relatively early-stage lung cancer, but who were otherwise unfit for surgery.[159] In 1997, continuous hyperfractionated accelerated radiotherapy was seen as an improvement over conventional radical radiotherapy.[160] With small-cell lung carcinoma, initial attempts in the 1960s at surgical resection[161] and radical radiotherapy[162] were unsuccessful. In the 1970s, successful chemotherapy regimens were developed.[163]
Research directions[edit]
Current research directions for lung cancer treatment include immunotherapy,[164] which encourages the body's immune system to attack the tumour cells, epigenetics, and new combinations of chemotherapy and radiotherapy, both on their own and together. Many of these new treatments work through immune checkpoint blockade, disrupting cancer's ability to evade the immune system.[164]
Ipilimumab blocks signaling through a receptor on T cells known as CTLA-4 which dampens down the immune system. It has been approved by the U.S. Food and Drug Administration (FDA) for treatment of melanoma and is undergoing clinical trials for both non-small cell lung cancer (NSCLC) and small cell lung cancer (SCLC).[164]
Other immunotherapy treatments interfere with the binding of programmed cell death 1 (PD-1) protein with its ligand PD-1 ligand 1 (PD-L1). Signaling through PD-1 inactivates T cells. Some cancer cells appear to exploit this by expressing PD-L1 in order to switch off T cells that might recognise them as a threat. Monoclonal antibodies targeting both PD-1 and PD-L1, such as pembrolizumab and nivolumab[165] are currently in clinical trials for treatment for lung cancer.[164]
Epigenetics is the study of small, usually heritable, molecular modifications – or ‘tags’- that bind DNA and modify gene expression levels. Targeting these ‘tags’ with drugs can kill cancer cells. Early-stage research in NSCLC using drugs aimed at epigenetic modifications shows that blocking more than one of these ‘tags’ can kill cancer cells with fewer side effects.[166] Studies also show that giving patients these drugs before standard treatment can improve its effectiveness. Clinical trials are underway to evaluate how well these drugs kill lung cancer cells in humans.[166] Several drugs that target epigenetic mechanisms are in development. Histone deacetylase inhibitors in development include valproic acid, vorinostat, belinostat, panobinostat, entinostat, and romidepsin. DNA methyltransferase inhibitors in development include decitabine, azacytidine, and hydralazine.[52]
The TRACERx project is looking at how NSCLC develops and evolves, and how these tumours become resistant to treatment.[167] The project will look at tumour samples from 850 NSCLC patients at various stages including diagnosis, after first treatment, post-treatment, and relapse.[168] By studying samples at different points of tumour development, the researchers hope to identify the changes that drive tumour growth and resistance to treatment. The results of this project will help scientists and doctors gain a better understanding of NSCLC and potentially lead to the development of new treatments against the disease.[167]
For lung cancer cases that develop resistance to epidermal growth factor receptor (EGFR) and anaplastic lymphoma kinase (ALK) tyrosine kinase inhibitors, new drugs are in development. New EGFR inhibitors include afatinib and dacomitinib. An alternative signaling pathway, c-Met, can be inhibited by tivantinib and onartuzumab. New ALK inhibitors include crizotinib and ceritinib.[169]
References[edit]
- ^ a b c d e f g h i j k l m n o p q r s t u v w x y z Horn, L; Pao W; Johnson DH (2012). "Chapter 89". In Longo, DL; Kasper, DL; Jameson, JL; Fauci, AS; Hauser, SL; Loscalzo, J (eds.). Harrison's Principles of Internal Medicine (18th ed.). McGraw-Hill. ISBN 0-07-174889-X.
- ^ a b c "Lung Carcinoma: Tumors of the Lungs". Merck Manual Professional Edition, Online edition. Retrieved 15 August 2007.
- ^ Thun MJ, Hannan LM, Adams-Campbell LL, et al. (September 2008). "Lung cancer occurrence in never-smokers: an analysis of 13 cohorts and 22 cancer registry studies". PLoS Medicine. 5 (9): e185. doi:10.1371/journal.pmed.0050185. PMC 2531137. PMID 18788891.
{{cite journal}}: CS1 maint: unflagged free DOI (link) - ^ a b c d e f g h Alberg AJ, Samet JM (2010). "Chapter 46". Murray & Nadel's Textbook of Respiratory Medicine (5th ed.). Saunders Elsevier. ISBN 978-1-4160-4710-0.
- ^ a b O'Reilly, KM; Mclaughlin AM; Beckett WS; Sime PJ (March 2007). "Asbestos-related lung disease". American Family Physician. 75 (5): 683–688. PMID 17375514.
- ^ Carmona, RH (27 June 2006). "The Health Consequences of Involuntary Exposure to Tobacco Smoke: A Report of the Surgeon General". U.S. Department of Health and Human Services.
Secondhand smoke exposure causes disease and premature death in children and adults who do not smoke.
Retrieved 2014-06-16 - ^ "Tobacco Smoke and Involuntary Smoking" (PDF). IARC Monographs on the Evaluation of Carcinogenic Risks to Humans. 83. WHO International Agency for Research on Cancer. 2002.
There is sufficient evidence that involuntary smoking (exposure to secondhand or 'environmental' tobacco smoke) causes lung cancer in humans. ... Involuntary smoking (exposure to secondhand or 'environmental' tobacco smoke) is carcinogenic to humans (Group 1).
- ^ a b c d e f g h i j k Lu C, Onn A, Vaporciyan AA, et al. (2010). "78: Cancer of the Lung". Holland-Frei Cancer Medicine (8th ed.). People's Medical Publishing House. ISBN 978-1-60795-014-1.
- ^ Chapman, S; Robinson G; Stradling J; West S (2009). "Chapter 31". Oxford Handbook of Respiratory Medicine (2nd ed.). Oxford University Press. ISBN 978-0-19-954516-2.
- ^ a b "Surveillance, Epidemiology and End Results Program". National Cancer Institute. Retrieved 15 July 2014.
- ^ a b World Cancer Report 2014. World Health Organization. 2014. pp. Chapter 1.1. ISBN 9283204298.
- ^ Honnorat, J; Antoine JC (May 2007). "Paraneoplastic neurological syndromes". Orphanet Journal of Rare Diseases. 2 (1). BioMed Central: 22. doi:10.1186/1750-1172-2-22. PMC 1868710. PMID 17480225.
{{cite journal}}: CS1 maint: unflagged free DOI (link) - ^ a b Greene, Frederick L. (2002). AJCC cancer staging manual. Berlin: Springer-Verlag. ISBN 0-387-95271-3.
- ^ a b c d e Collins, LG; Haines C; Perkel R; Enck RE (January 2007). "Lung cancer: diagnosis and management". American Family Physician. 75 (1). American Academy of Family Physicians: 56–63. PMID 17225705.
- ^ Brown KM, Keats JJ, Sekulic A, et al. (2010). "Chapter 8". Holland-Frei Cancer Medicine (8th ed.). People's Medical Publishing House USA. ISBN 978-1-60795-014-1.
- ^ Biesalski HK, Bueno de Mesquita B, Chesson A, et al. (1998). "European Consensus Statement on Lung Cancer: risk factors and prevention. Lung Cancer Panel". CA Cancer J Clin. 48 (3). Smoking is the major risk factor, accounting for about 90% of lung cancer incidence.: 167–176, discussion 164–166. doi:10.3322/canjclin.48.3.167. PMID 9594919.
{{cite journal}}: CS1 maint: location (link) - ^ a b Hecht, SS (2012). "Lung carcinogenesis by tobacco smoke". International Journal of Cancer. 131 (12): 2724–2732. doi:10.1002/ijc.27816. PMC 3479369. PMID 22945513.
- ^ Kumar, V; Abbas AK; Aster JC (2013). "Chapter 5". Robbins Basic Pathology (9th ed.). Elsevier Saunders. p. 199. ISBN 978-1-4377-1781-5.
- ^ Peto R, Lopez AD, Boreham J, et al. (2006). Mortality from smoking in developed countries 1950–2000: Indirect estimates from National Vital Statistics. Oxford University Press. ISBN 0-19-262535-7.
- ^ California Environmental Protection Agency (1997). "Health effects of exposure to environmental tobacco smoke. California Environmental Protection Agency". Tobacco Control. 6 (4): 346–353. doi:10.1136/tc.6.4.346. PMC 1759599. PMID 9583639.
- ^ Centers for Disease Control and Prevention (CDC) (December 2001). "State-specific prevalence of current cigarette smoking among adults, and policies and attitudes about secondhand smoke—United States, 2000". Morbidity and Mortality Weekly Report. 50 (49). Atlanta, Georgia: CDC: 1101–1106. PMID 11794619.
- ^ a b c Alberg, AJ; Samet JM (September 2007). "Epidemiology of lung cancer". Chest. 132 (S3). American College of Chest Physicians: 29S–55S. doi:10.1378/chest.07-1347. PMID 17873159.
- ^ Jaakkola, MS; Jaakkola JJ (August 2006). "Impact of smoke-free workplace legislation on exposures and health: possibilities for prevention". European Respiratory Journal. 28 (2): 397–408. doi:10.1183/09031936.06.00001306. PMID 16880370.
- ^ Parkin, DM (December 2011). "Tobacco—attributable cancer burden in the UK in 2010". British Journal of Cancer. 105 (Suppl. 2): S6–S13. doi:10.1038/bjc.2011.475. PMC 3252064. PMID 22158323.
- ^ a b Taylor, R; Najafi F; Dobson A (October 2007). "Meta-analysis of studies of passive smoking and lung cancer: effects of study type and continent". International Journal of Epidemiology. 36 (5): 1048–1059. doi:10.1093/ije/dym158. PMID 17690135.
- ^ "Frequently asked questions about second hand smoke". World Health Organization. Retrieved 25 July 2012.
- ^ Schick, S; Glantz S (December 2005). "Philip Morris toxicological experiments with fresh sidestream smoke: more toxic than mainstream smoke". Tobacco Control. 14 (6): 396–404. doi:10.1136/tc.2005.011288. PMC 1748121. PMID 16319363.
- ^ Greydanus, DE; Hawver EK; Greydanus MM (October 2013). "Marijuana: current concepts". Frontiers in Public Health. 1 (42). doi:10.3389/fpubh.2013.00042. PMC 3859982. PMID 24350211.
{{cite journal}}: CS1 maint: unflagged free DOI (link) - ^ Owen, KP; Sutter, ME; Albertson, TE (February 2014). "Marijuana: respiratory tract effects". Clinical reviews in allergy & immunology. 46 (1): 65–81. doi:10.1007/s12016-013-8374-y. PMID 23715638.
- ^ Joshi, M; Joshi, A; Bartter, T (March 2014). "Marijuana and lung diseases". Current Opinion in Pulmonary Medicine. 20 (2): 173–179. doi:10.1097/mcp.0000000000000026. PMID 24384575.
- ^ Tashkin, DP (June 2013). "Effects of marijuana smoking on the lung". Annals of the American Thoracic Society. 10 (3): 239–47. doi:10.1513/annalsats.201212-127fr. PMID 23802821.
- ^ Underner, M; Urban T; Perriot J (June 2014). "Cannabis smoking and lung cancer". Revue des Maladies Respiratoires. 31 (6): 488–498. doi:10.1016/j.rmr.2013.12.002. PMID 25012035.
- ^ Choi, H; Mazzone, P (September 2014). "Radon and lung cancer: assessing and mitigating the risk". Cleveland Clinic Journal of Medicine. 81 (9): 567–575. doi:10.3949/ccjm.81a.14046. PMID 25183848.
- ^ "Radon (Rn) Health Risks". EPA.
- ^ Schmid K, Kuwert T, Drexler H (March 2010). "Radon in Indoor Spaces: An Underestimated Risk Factor for Lung Cancer in Environmental Medicine". Dtsch Arztebl Int. 107 (11): 181–6. doi:10.3238/arztebl.2010.0181. PMC 2853156. PMID 20386676.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ EPA (February 2013). "Radiation information: radon". EPA.
- ^ Tobias, J; Hochhauser D (2010). "Chapter 12". Cancer and its Management (6th ed.). Wiley-Blackwell. p. 199. ISBN 978-1405-170154.
- ^ Davies, RJO; Lee YCG (2010). "18.19.3". Oxford Textbook Medicine (5th ed.). OUP Oxford. ISBN 978-0-19-920485-4.
- ^ Chen, H; Goldberg MS; Villeneuve PJ (October–December 2008). "A systematic review of the relation between long-term exposure to ambient air pollution and chronic diseases". Reviews on Environmental Health. 23 (4): 243–297. doi:10.1515/reveh.2008.23.4.243. PMID 19235364.
- ^ Clapp, RW; Jacobs MM; Loechler EL (January–March 2008). "Environmental and Occupational Causes of Cancer New Evidence, 2005–2007". Reviews on Environmental Health. 23 (1): 1–37. doi:10.1515/REVEH.2008.23.1.1. PMC 2791455. PMID 18557596.
- ^ a b Lim, WY; Seow, A (January 2012). "Biomass fuels and lung cancer". Respirology (Carlton, Vic.). 17 (1): 20–31. doi:10.1111/j.1440-1843.2011.02088.x. PMID 22008241.
- ^ a b Sood, A (December 2012). "Indoor fuel exposure and the lung in both developing and developed countries: an update". Clinics in chest medicine. 33 (4): 649–65. doi:10.1016/j.ccm.2012.08.003. PMID 23153607.
- ^ Yang, IA; Holloway, JW; Fong, KM (October 2013). "Genetic susceptibility to lung cancer and co-morbidities". Journal of Thoracic Disease. 5 (Suppl. 5): S454–S462. doi:10.3978/j.issn.2072-1439.2013.08.06. PMC 3804872. PMID 24163739.
- ^ Kern JA, McLennan G (2008). Fishman's Pulmonary Diseases and Disorders (4th ed.). McGraw-Hill. p. 1802. ISBN 0-07-145739-9.
- ^ Larsen, JE; Minna D (December 2011). "Molecular biology of lung cancer: clinical implications". Clinics in Chest Medicine. 32 (4): 703–740. doi:10.1016/j.ccm.2011.08.003. PMC 3367865. PMID 22054881.
- ^ "Preventable exposures associated with human cancers" (PDF). Journal of the National Cancer Institute. 103 (24): 1827–39. 21 December 2011. doi:10.1093/jnci/djr483. PMID 22158127.
{{cite journal}}: Cite uses deprecated parameter|authors=(help) - ^ Cooper, WA; Lam DLC; O'Toole SA (October 2013). "Molecular biology of lung cancer" (PDF). Journal of Thoracic Disease. 5 (Suppl. 5): S. 479–490. doi:10.3978/j.issn.2072-1439.2013.08.03. PMC 3804875. PMID 24163741.
- ^ Tobias, J; Hochhauser D (2010). "Chapter 12". Cancer and its Management (6th ed.). Wiley-Blackwell. p. 200. ISBN 978-1405-170154.
- ^ a b c d Herbst, RS; Heymach JV; Lippman SM (September 2008). "Lung cancer". New England Journal of Medicine. 359 (13): 1367–1380. doi:10.1056/NEJMra0802714. PMID 18815398.
- ^ Aviel-Ronen, S; Blackhall FH; Shepherd FA; Tsao MS (July 2006). "K-ras mutations in non-small-cell lung carcinoma: a review". Clinical Lung Cancer. 8 (1). Cancer Information Group: 30–38. doi:10.3816/CLC.2006.n.030. PMID 16870043.
- ^ Kumar, V; Abbas AK; Aster JC (2013). "Chapter 5". Robbins Basic Pathology (9th ed.). Elsevier Saunders. p. 212. ISBN 978-1-4377-1781-5.
- ^ a b Jakopovic, M; Thomas A; Balasubramaniam S (October 2013). "Targeting the epigenome in lung cancer: expanding approaches to epigenetic therapy" (PDF). Frontiers in Oncology. 3 (261). doi:10.3389/fonc.2013.00261. PMC 3793201. PMID 24130964.
{{cite journal}}: CS1 maint: unflagged free DOI (link) - ^ a b Mulvihill, MS; Kratz JR; Pham P (February 2013). "The role of stem cells in airway repair: implications for the origins of lung cancer". Chinese Journal of Cancer. 32 (2): 71–74. doi:10.5732/cjc.012.10097. PMC 3845611. PMID 23114089.
- ^ Powell, CA; Halmos B; Nana-Sinkam SP (July 2013). "Update in lung cancer and mesothelioma 2012" (PDF). American Journal of Respiratory and Critical Care Medicine. 188 (2): 157–166. doi:10.1164/rccm.201304-0716UP. PMC 3778761. PMID 23855692.
- ^ Miller, WT (2008). Fishman's Pulmonary Diseases and Disorders (4th ed.). McGraw-Hill. p. 486. ISBN 0-07-145739-9.
- ^ Kaiser, LR (2008). Fishman's Pulmonary Diseases and Disorders (4th ed.). McGraw-Hill. pp. 1815–1816. ISBN 0-07-145739-9.
- ^ a b c d e American College of Chest Physicians; American Thoracic Society (September 2013), "Five Things Physicians and Patients Should Question", Choosing Wisely: an initiative of the ABIM Foundation, American College of Chest Physicians and American Thoracic Society, retrieved 6 January 2013
- ^ a b Kumar, V; Abbas AK; Aster JC (2013). "12". Robbins Basic Pathology (9th ed.). Elsevier Saunders. p. 505. ISBN 978-1-4377-1781-5.
- ^ Subramanian, J; Govindan R (February 2007). "Lung cancer in never smokers: a review". Journal of Clinical Oncology. 25 (5). American Society of Clinical Oncology: 561–570. doi:10.1200/JCO.2006.06.8015. PMID 17290066.
- ^ Raz, DJ; He B; Rosell R; Jablons DM (March 2006). "Bronchioloalveolar carcinoma: a review". Clinical Lung Cancer. 7 (5): 313–322. doi:10.3816/CLC.2006.n.012. PMID 16640802.
- ^ Rosti G, Bevilacqua G, Bidoli P, et al. (March 2006). "Small cell lung cancer". Annals of Oncology. 17 (Suppl. 2): 5–10. doi:10.1093/annonc/mdj910. PMID 16608983.
- ^ Seo JB, Im JG, Goo JM, et al. (1 March 2001). "Atypical pulmonary metastases: spectrum of radiologic findings". Radiographics. 21 (2): 403–417. doi:10.1148/radiographics.21.2.g01mr17403. PMID 11259704.
- ^ Tan D, Zander DS (2008). "Immunohistochemistry for Assessment of Pulmonary and Pleural Neoplasms: A Review and Update". Int J Clin Exp Pathol. 1 (1): 19–31. PMC 2480532. PMID 18784820.
- ^ Chheang, S; Brown K (June 2013). "Lung cancer staging: clinical and radiologic perspectives". Seminars in Interventional Radiology. 30 (2): 99–113. doi:10.1055/s-0033-1342950. PMC 3709937. PMID 24436525.
- ^ a b c Rami-Porta, R; Crowley JJ; Goldstraw P (February 2009). "The revised TNM staging system for lung cancer" (PDF). Annals of Thoracic and Cardiovascular Surgery. 15 (1): 4–9. PMID 19262443.
- ^ Dela Cruz, CS; Tanoue LT; Matthay RA (December 2011). "Lung cancer: epidemiology, etiology, and prevention" (PDF). Clinic in Chest Medicine. 32 (4): 605–644. doi:10.1016/j.ccm.2011.09.001. PMC 3864624. PMID 22054876.
- ^ Goodman, GE (November 2002). "Lung cancer. 1: prevention of lung cancer" (PDF). Thorax. 57 (11): 994–999. doi:10.1136/thorax.57.11.994. PMC 1746232. PMID 12403886.
- ^ McNabola, A; Gill LW (February 2009). "The control of environmental tobacco smoke: a policy review". International Journal of Environmental Research and Public Health. 6 (2): 741–758. doi:10.3390/ijerph6020741. PMC 2672352. PMID 19440413.
{{cite journal}}: CS1 maint: unflagged free DOI (link) - ^ Pandey, G (February 2005). "Bhutan's smokers face public ban". BBC. Retrieved 7 September 2007.
- ^ Pandey, G (2 October 2008). "Indian ban on smoking in public". BBC. Retrieved 25 April 2012.
- ^ "UN health agency calls for total ban on tobacco advertising to protect young" (Press release). United Nations News service. 30 May 2008.
- ^ Gutierrez, A; Suh R; Abtin F (June 2013). "Lung cancer screening". Seminars in Interventional Radiology. 30 (2): 114–120. doi:10.1055/s-0033-1342951. PMC 3709936. PMID 24436526.
- ^ Jaklitsch MT, Jacobson FL, Austin JH, et al. (July 2012). "The American Association for Thoracic Surgery guidelines for lung cancer screening using low-dose computed tomography scans for lung cancer survivors and other high-risk groups". Journal of Thoracic and Cardiovascular Surgery. 144 (1): 33–38. doi:10.1016/j.jtcvs.2012.05.060. PMID 22710039.
- ^ Bach PB, Mirkin JN, Oliver TK, et al. (June 2012). "Benefits and harms of CT screening for lung cancer: a systematic review". JAMA: the Journal of the American Medical Association. 307 (22): 2418–2429. doi:10.1001/jama.2012.5521. PMC 3709596. PMID 22610500.
- ^ a b Aberle, D. R.; Abtin, F.; Brown, K. (2013). "Computed Tomography Screening for Lung Cancer: Has It Finally Arrived? Implications of the National Lung Screening Trial". Journal of Clinical Oncology. 31 (8): 1002–1008. doi:10.1200/JCO.2012.43.3110. ISSN 0732-183X.
- ^ Bach PB, Mirkin JN, Oliver TK, et al. (June 2012). "Benefits and harms of CT screening for lung cancer: a systematic review". JAMA. 307 (22): 2418–29. doi:10.1001/jama.2012.5521. PMC 3709596. PMID 22610500.
- ^ "Screening for lung cancer". Cochrane Database of Systematic Reviews (6): CD001991. 2013. doi:10.1002/14651858.CD001991.pub3. PMID 23794187.
{{cite journal}}: Cite uses deprecated parameter|authors=(help) - ^ a b Moyer, VA; U.S. Preventive Services Task, Force (4 March 2014). "Screening for lung cancer: U.S. Preventive Services Task Force recommendation statement". Annals of internal medicine. 160 (5): 330–8. doi:10.7326/M13-2771. PMID 24378917.
- ^ Baldwin, DR; Hansell, DM; Duffy, SW; Field, JK (7 March 2014). "Lung cancer screening with low dose computed tomography". BMJ (Clinical research ed.). 348: g1970. doi:10.1136/bmj.g1970. PMID 24609921.
- ^ a b c Fabricius, P; Lange P (July–September 2003). "Diet and lung cancer". Monaldi Archives for Chest Disease. 59 (3): 207–211. PMID 15065316.
- ^ Fritz H, Kennedy D, Fergusson D, et al. (2011). "Vitamin A and Retinoid Derivatives for Lung Cancer: A Systematic Review and Meta Analysis". PLoS ONE. 6 (6): e21107. doi:10.1371/journal.pone.0021107. PMC 3124481. PMID 21738614.
{{cite journal}}: CS1 maint: unflagged free DOI (link) - ^ Herr C, Greulich T, Koczulla RA, et al. (March 2011). "The role of vitamin D in pulmonary disease: COPD, asthma, infection, and cancer". Respiratory Research. 12 (1): 31. doi:10.1186/1465-9921-12-31. PMC 3071319. PMID 21418564.
{{cite journal}}: CS1 maint: unflagged free DOI (link) - ^ a b Key, TJ (January 2011). "Fruit and vegetables and cancer risk". British Journal of Cancer. 104 (1): 6–11. doi:10.1038/sj.bjc.6606032. PMC 3039795. PMID 21119663.
- ^ Ferrell, B; Koczywas M; Grannis F; Harrington A (April 2011). "Palliative care in lung cancer". Surgical Clinics of North America. 91 (2): 403–417. doi:10.1016/j.suc.2010.12.003. PMID 21419260.
- ^ Kaiser LR (2008). Fishman's Pulmonary Diseases and Disorders (4th ed.). McGraw-Hill. pp. 1853–1854. ISBN 0-07-145739-9.
- ^ a b Kaiser LR (2008). Fishman's Pulmonary Diseases and Disorders (4th ed.). McGraw-Hill. pp. 1855–1856. ISBN 0-07-145739-9.
- ^ Odell, DD; Kent MS; Fernando HC (Spring 2010). "Sublobar resection with brachytherapy mesh for stage I non-small cell lung cancer". Seminars in Thoracic and Cardiovascular Surgery. 22 (1): 32–37. doi:10.1053/j.semtcvs.2010.04.003. PMID 20813314.
- ^ Alam, N; Flores RM (July–September 2007). "Video-assisted thoracic surgery (VATS) lobectomy: the evidence base". Journal of the Society of Laparoendoscopic Surgeons. 11 (3): 368–374. PMC 3015831. PMID 17931521.
- ^ Rueth, NM; Andrade RS (June 2010). "Is VATS lobectomy better: perioperatively, biologically and oncologically?". Annals of Thoracic Surgery. 89 (6): S2107–S2111. doi:10.1016/j.athoracsur.2010.03.020. PMID 20493991.
- ^ Simon GR, Turrisi A (September 2007). "Management of small cell lung cancer: ACCP evidence-based clinical practice guidelines (2nd edition)". Chest. 132 (3 Suppl): 324S–339S. doi:10.1378/chest.07-1385. PMID 17873178.
- ^ Goldstein, SD; Yang SC (October 2011). "Role of surgery in small cell lung cancer". Surgical Oncology Clinics of North America. 20 (4): 769–777. doi:10.1016/j.soc.2011.08.001. PMID 21986271.
- ^ Arriagada, R; Goldstraw P; Le Chevalier T (2002). Oxford Textbook of Oncology (2nd ed.). Oxford University Press. p. 2094. ISBN 0-19-262926-3.
- ^ Hatton, MQ; Martin JE (June 2010). "Continuous hyperfractionated accelerated radiotherapy (CHART) and non-conventionally fractionated radiotherapy in the treatment of non-small cell lung cancer: a review and consideration of future directions". Clinical Oncology (Royal College of Radiologists). 22 (5): 356–364. doi:10.1016/j.clon.2010.03.010. PMID 20399629.
- ^ PORT Meta-analysis Trialists Group (2005). Rydzewska, Larysa (ed.). "Postoperative radiotherapy for non-small cell lung cancer". Cochrane Database of Systematic Reviews (2): CD002142. doi:10.1002/14651858.CD002142.pub2. PMID 15846628.
- ^ Le Péchoux, C (2011). "Role of postoperative radiotherapy in resected non-small cell lung cancer: a reassessment based on new data". Oncologist. 16 (5): 672–681. doi:10.1634/theoncologist.2010-0150. PMC 3228187. PMID 21378080.
- ^ Ikushima, H (February 2010). "Radiation therapy: state of the art and the future". Journal of Medical Investigation. 57 (1–2): 1–11. doi:10.2152/jmi.57.1. PMID 20299738.
- ^ Reveiz, L; Rueda, JR; Cardona, AF (12 December 2012). "Palliative endobronchial brachytherapy for non-small cell lung cancer". The Cochrane database of systematic reviews. 12: CD004284. PMID 23235606.
- ^ Paumier, A; Cuenca X; Le Péchoux C (June 2011). "Prophylactic cranial irradiation in lung cancer". Cancer Treatment Reviews. 37 (4): 261–265. doi:10.1016/j.ctrv.2010.08.009. PMID 20934256.
- ^ Girard, N; Mornex F (October 2011). "Stereotactic radiotherapy for non-small cell lung cancer: From concept to clinical reality. 2011 update". Cancer Radiothérapie. 15 (6–7): 522–526. doi:10.1016/j.canrad.2011.07.241. PMID 21889901.
- ^ Fairchild A, Harris K, Barnes E, et al. (August 2008). "Palliative thoracic radiotherapy for lung cancer: a systematic review". Journal of Clinical Oncology. 26 (24): 4001–4011. doi:10.1200/JCO.2007.15.3312. PMID 18711191.
- ^ Hann CL, Rudin CM (30 November 2008). "Management of small-cell lung cancer: incremental changes but hope for the future". Oncology (Williston Park). 22 (13): 1486–92. PMID 19133604.
- ^ Murray, N; Turrisi AT (March 2006). "A review of first-line treatment for small-cell lung cancer". Journal of Thoracic Oncology. 1 (3): 270–278. PMID 17409868.
- ^ Azim, HA; Ganti AK (March 2007). "Treatment options for relapsed small-cell lung cancer". Anticancer drugs. 18 (3): 255–261. doi:10.1097/CAD.0b013e328011a547. PMID 17264756.
- ^ MacCallum, C; Gillenwater HH (July 2006). "Second-line treatment of small-cell lung cancer". Current Oncology Reports. 8 (4): 258–264. doi:10.1007/s11912-006-0030-8. PMID 17254525.
- ^ a b NSCLC Meta-Analyses Collaborative Group (October 2008). "Chemotherapy in Addition to Supportive Care Improves Survival in Advanced Non–Small-Cell Lung Cancer: A Systematic Review and Meta-Analysis of Individual Patient Data From 16 Randomized Controlled Trials". J. Clin. Oncol. 26 (28): 4617–25. doi:10.1200/JCO.2008.17.7162. PMC 2653127. PMID 18678835.
- ^ Mehra R, Treat J (2008). Fishman's Pulmonary Diseases and Disorders (4th ed.). McGraw-Hill. p. 1876. ISBN 0-07-145739-9.
- ^ a b Clegg A, Scott DA, Hewitson P, et al. (January 2002). "Clinical and cost effectiveness of paclitaxel, docetaxel, gemcitabine, and vinorelbine in non-small cell lung cancer: a systematic review". Thorax. 57 (1). BMJ Publishing Group: 20–28. doi:10.1136/thorax.57.1.20. PMC 1746188. PMID 11809985.
- ^ Fuld AD, Dragnev KH, Rigas JR (June 2010). "Pemetrexed in advanced non-small-cell lung cancer". Expert Opin Pharmacother. 11 (8): 1387–402. doi:10.1517/14656566.2010.482560. PMID 20446853.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Carbone, DP; Felip E (September 2011). "Adjuvant therapy in non-small cell lung cancer: future treatment prospects and paradigms". Clinical Lung Cancer. 12 (5): 261–271. doi:10.1016/j.cllc.2011.06.002. PMID 21831720.
- ^ a b Le Chevalier, T (October 2010). "Adjuvant chemotherapy for resectable non-small-cell lung cancer: where is it going?". Annals of Oncology. 21 (Suppl. 7): vii196–198. doi:10.1093/annonc/mdq376. PMID 20943614.
- ^ Horn, L; Sandler AB; Putnam JB Jr; Johnson DH (May 2007). "The rationale for adjuvant chemotherapy in stage I non-small cell lung cancer". Journal of Thoracic Oncology. 2 (5): 377–383. doi:10.1097/01.JTO.0000268669.64625.bb. PMID 17473651.
- ^ Wakelee, HA; Schiller JH; Gandara DR (July 2006). "Current status of adjuvant chemotherapy for stage IB non-small-cell lung cancer: implications for the New Intergroup Trial". Clinical Lung Cancer. 8 (1). Cancer Information Group: 18–21. doi:10.3816/CLC.2006.n.028. PMID 16870041.
- ^ NSCLC Meta-analysis Collaborative, Group (3 May 2014). "Preoperative chemotherapy for non-small-cell lung cancer: a systematic review and meta-analysis of individual participant data". Lancet. 383 (9928): 1561–71. doi:10.1016/S0140-6736(13)62159-5. PMC 4022989. PMID 24576776.
- ^ Souquet PJ, Chauvin F, Boissel JP, Bernard JP (April 1995). "Meta-analysis of randomised trials of systemic chemotherapy versus supportive treatment in non-resectable non-small cell lung cancer". Lung Cancer. 12 Suppl 1: S147–54. doi:10.1016/0169-5002(95)00430-9. PMID 7551923.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Sörenson S, Glimelius B, Nygren P (2001). "A systematic overview of chemotherapy effects in non-small cell lung cancer". Acta Oncologica. 40 (2–3): 327–39. doi:10.1080/02841860151116402. PMID 11441939.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Clegg A, Scott DA, Sidhu M, Hewitson P, Waugh N (2001). "A rapid and systematic review of the clinical effectiveness and cost-effectiveness of paclitaxel, docetaxel, gemcitabine and vinorelbine in non-small-cell lung cancer". Health Technology Assessment. 5 (32): 1–195. PMID 12065068.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Non-Small Cell Lung Cancer Collaborative, Group (12 May 2010). "Chemotherapy and supportive care versus supportive care alone for advanced non-small cell lung cancer". The Cochrane database of systematic reviews (5): CD007309. doi:10.1002/14651858.CD007309.pub2. PMID 20464750.
- ^ D'Antonio; Passaro A; Gori B (May 2014). "Bone and brain metastasis in lung cancer: recent advances in therapeutic strategies". Therapeutic Advances in Medical Oncology. 6 (3): 101–114. doi:10.1177/1758834014521110. PMC 3987652. PMID 24790650.
- ^ Parikh, RB; Kirch, RA; Smith, TJ; Temel, JS (12 December 2013). "Early specialty palliative care--translating data in oncology into practice". The New England Journal of Medicine. 369 (24): 2347–51. doi:10.1056/nejmsb1305469. PMC 3991113. PMID 24328469.
- ^ Kelley AS, Meier DE (August 2010). "Palliative care—a shifting paradigm". New England Journal of Medicine. 363 (8): 781–2. doi:10.1056/NEJMe1004139. PMID 20818881.
- ^ a b Prince-Paul M (April 2009). "When hospice is the best option: an opportunity to redefine goals". Oncology (Williston Park, N.Y.). 23 (4 Suppl Nurse Ed): 13–7. PMID 19856592.
- ^ Ridge, CA; McErlean AM; Ginsberg MS (June 2013). "Epidemiology of lung cancer". Seminars in Interventional Radiology. 30 (2): 93–98. doi:10.1055/s-0033-1342949. PMC 3709917. PMID 24436524.
- ^ "Lung cancer survival statistics". Cancer Research UK.
- ^ Majumder, edited by Sadhan (2009). Stem cells and cancer (Online-Ausg. ed.). New York: Springer. p. 193. ISBN 978-0-387-89611-3.
{{cite book}}:|first=has generic name (help) - ^ "Lung cancer survival statistics". Retrieved 28 October 2014.
- ^ "Non-Small Cell Lung Cancer Treatment". PDQ for Health Professionals. National Cancer Institute. Retrieved 22 November 2008.
- ^ "Small Cell Lung Cancer Treatment". PDQ for Health Professionals. National Cancer Institute. 2012. Retrieved 16 May 2012.
- ^ Spiro, SG (2010). "18.19.1". Oxford Textbook Medicine (5th ed.). OUP Oxford. ISBN 978-0-19-920485-4.
- ^ SEER data (SEER.cancer.gov) Median Age of Cancer Patients at Diagnosis 2002–2003
- ^ SEER data (SEER.cancer.gov) Median Age of Cancer Patients at Death 2002–2006
- ^ Slatore, CG; Au DH; Gould MK (November 2010). "An official American Thoracic Society systematic review: insurance status and disparities in lung cancer practices and outcomes". American Journal of Respiratory and Critical Care Medicine. 182 (9): 1195–1205. doi:10.1164/rccm.2009-038ST. PMID 21041563.
- ^ "WHO Disease and injury country estimates". World Health Organization. 2009. Retrieved 11 November 2009.
- ^ Stewart, edited by Bernard W.; Wild, Christopher P. (2014). World cancer report 2014. Lyon: IARC Press. pp. 350–352. ISBN 9789283204299.
{{cite book}}:|first1=has generic name (help) - ^ Jemal A, Tiwari RC, Murray T, et al. (2004). "Cancer statistics, 2004". CA: a Cancer Journal for Clinicians. 54 (1): 8–29. doi:10.3322/canjclin.54.1.8. PMID 14974761.
- ^ Proctor, RN (March 2012). "The history of the discovery of the cigarette-lung cancer link: evidentiary traditions, corporate denial, global toll". Tobacco Control. 21 (2): 87–91. doi:10.1136/tobaccocontrol-2011-050338. PMID 22345227.
- ^ Lum, KL; Polansky JR; Jackler RK; Glantz SA (October 2008). "Signed, sealed and delivered: "big tobacco" in Hollywood, 1927–1951". Tobacco Control. 17 (5): 313–323. doi:10.1136/tc.2008.025445. PMC 2602591. PMID 18818225.
- ^ Lovato, C; Watts A; Stead LF (October 2011). "Impact of tobacco advertising and promotion on increasing adolescent smoking behaviours". Cochrane Database of Systematic Reviews (10): CD003439. doi:10.1002/14651858.CD003439.pub2. PMID 21975739.
- ^ Kemp, FB (July–September 2009). "Smoke free policies in Europe. An overview". Pneumologia. 58 (3): 155–158. PMID 19817310.
- ^ National Cancer Institute; SEER stat fact sheets: Lung and Bronchus. Surveillance Epidemiology and End Results. 2010 [1]
- ^ "Gender in lung cancer and smoking research" (PDF). World Health Organization. 2004. Retrieved 26 May 2007.
- ^ Zhang, J; Ou JX; Bai CX (November 2011). "Tobacco smoking in China: prevalence, disease burden, challenges and future strategies". Respirology. 16 (8): 1165–1172. doi:10.1111/j.1440-1843.2011.02062.x. PMID 21910781.
- ^ Behera, D; Balamugesh T (2004). "Lung cancer in India" (PDF). Indian Journal of Chest Diseases and Allied Sciences. 46 (4): 269–281. PMID 15515828.
- ^ "Lung cancer statistics". Cancer Research UK. Retrieved 28 October 2014.
- ^ Charloux A, Quoix E, Wolkove N, et al. (February 1997). "The increasing incidence of lung adenocarcinoma: reality or artefact? A review of the epidemiology of lung adenocarcinoma". International Journal of Epidemiology. 26 (1): 14–23. doi:10.1093/ije/26.1.14. PMID 9126499.
- ^ Kadara, H; Kabbout M; Wistuba II (January 2012). "Pulmonary adenocarcinoma: a renewed entity in 2011". Respirology. 17 (1): 50–65. doi:10.1111/j.1440-1843.2011.02095.x. PMID 22040022.
- ^ Morgagni, Giovanni Battista (1761). De sedibus et causis morborum per anatomen indagatis. OL 24830495M.
- ^ Bayle, Gaspard-Laurent (1810). Recherches sur la phthisie pulmonaire (in French). Paris. OL 15355651W.
- ^ a b Witschi, H (November 2001). "A short history of lung cancer". Toxicological Sciences. 64 (1): 4–6. doi:10.1093/toxsci/64.1.4. PMID 11606795.
- ^ Adler, I (1912). Primary Malignant Growths of the Lungs and Bronchi. New York: Longmans, Green, and Company. OCLC 14783544. OL 24396062M., cited in Spiro SG, Silvestri GA (2005). "One hundred years of lung cancer". American Journal of Respiratory and Critical Care Medicine. 172 (5): 523–529. doi:10.1164/rccm.200504-531OE. PMID 15961694.
- ^ Grannis, FW. "History of cigarette smoking and lung cancer". smokinglungs.com. Archived from the original on 18 July 2007. Retrieved 6 August 2007.
- ^ Proctor, R (2000). The Nazi War on Cancer. Princeton University Press. pp. 173–246. ISBN 0-691-00196-0.
- ^ Doll, R; Hill AB (November 1956). "Lung Cancer and Other Causes of Death in Relation to Smoking". British Medical Journal. 2 (5001): 1071–1081. doi:10.1136/bmj.2.5001.1071. PMC 2035864. PMID 13364389.
- ^ US Department of Health Education and Welfare (1964). "Smoking and health: report of the advisory committee to the Surgeon General of the Public Health Service" (PDF). Washington, DC: US Government Printing Office.
- ^ a b Greaves, M (2000). Cancer: the Evolutionary Legacy. Oxford University Press. pp. 196–197. ISBN 0-19-262835-6.
- ^ Greenberg, M; Selikoff IJ (February 1993). "Lung cancer in the Schneeberg mines: a reappraisal of the data reported by Harting and Hesse in 1879". Annals of Occupational Hygiene. 37 (1): 5–14. doi:10.1093/annhyg/37.1.5. PMID 8460878.
- ^ Samet, JM (April 2011). "Radiation and cancer risk: a continuing challenge for epidemiologists". Environmental Health. 10 (Suppl. 1): S4. doi:10.1186/1476-069X-10-S1-S4. PMC 3073196. PMID 21489214.
{{cite journal}}: CS1 maint: unflagged free DOI (link) - ^ Horn, L; Johnson DH (July 2008). "Evarts A. Graham and the first pneumonectomy for lung cancer". Journal of Clinical Oncology. 26 (19): 3268–3275. doi:10.1200/JCO.2008.16.8260. PMID 18591561.
- ^ Edwards, AT (1946). "Carcinoma of the Bronchus". Thorax. 1 (1): 1–25. doi:10.1136/thx.1.1.1. PMC 1018207. PMID 20986395.
- ^ Kabela, M (1956). "Erfahrungen mit der radikalen Röntgenbestrahlung des Bronchienkrebses". Ceskoslovenská Onkológia (in German). 3 (2): 109–115. PMID 13383622.
{{cite journal}}: Unknown parameter|trans_title=ignored (|trans-title=suggested) (help) - ^ Saunders M, Dische S, Barrett A, et al. (July 1997). "Continuous hyperfractionated accelerated radiotherapy (CHART) versus conventional radiotherapy in non-small-cell lung cancer: a randomised multicentre trial". Lancet. 350 (9072). Elsevier: 161–165. doi:10.1016/S0140-6736(97)06305-8. PMID 9250182.
- ^ Lennox SC, Flavell G, Pollock DJ, et al. (November 1968). "Results of resection for oat-cell carcinoma of the lung". Lancet. 2 (7575). Elsevier: 925–927. doi:10.1016/S0140-6736(68)91163-X. PMID 4176258.
- ^ Miller, AB; Fox W; Tall R (September 1969). "Five-year follow-up of the Medical Research Council comparative trial of surgery and radiotherapy for the primary treatment of small-celled or oat-celled carcinoma of the bronchus". Lancet. 2 (7619). Elsevier: 501–505. doi:10.1016/S0140-6736(69)90212-8. PMID 4184834.
- ^ "Intensive chemotherapy of small cell bronchogenic carcinoma". Cancer Treatment Reports. 61 (3): 349–354. 1977. PMID 194691.
{{cite journal}}: Cite uses deprecated parameter|authors=(help) - ^ a b c d Brahmer, JR (February 2014). "Immune checkpoint blockade: the hope for immunotherapy as a treatment of lung cancer?". Seminars in oncology. 41 (1): 126–32. doi:10.1053/j.seminoncol.2013.12.014. PMID 24565586.
- ^ Powell, CA; Halmos, B; Nana-Sinkam, SP (July 2013). "Update in lung cancer and mesothelioma 2012". American Journal of Respiratory and Critical Care Medicine. 188 (2): 157–166. doi:10.1164/rccm.201304-0716UP. PMC 3778761. PMID 23855692.
- ^ a b Forde, PM; Brahmer, JR; Kelly, RJ (1 May 2014). "New strategies in lung cancer: epigenetic therapy for non-small cell lung cancer". Clinical Cancer Research. 20 (9): 2244–8. doi:10.1158/1078-0432.ccr-13-2088. PMID 24644000.
- ^ a b Jamal-Hanjani, M; Hackshaw, A; Ngai, Y; et al. (July 2014). "Tracking genomic cancer evolution for precision medicine: the lung TRACERx study". PLOS Biology. 12 (7): e1001906. doi:10.1371/journal.pbio.1001906. PMID 25003521.
{{cite journal}}: CS1 maint: unflagged free DOI (link) - ^ TRACERx project, Cancer Research UK science blog
- ^ Spaans, JN; Goss, GD (August 2014). "Trials to overcome drug resistance to EGFR and ALK targeted therapies—past, present, and future". Frontiers in Oncology. 4 (233). doi:10.3389/fonc.2014.00233. PMC 4145253. PMID 25221748.
{{cite journal}}: CS1 maint: unflagged free DOI (link)
External links[edit]