User:Bear919506/Sandbox
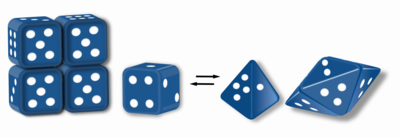
Morpheein – a protein that can form two or more different homo-oligomers (morpheein forms), but must come apart and change shape to convert between forms. The alternate shape may reassemble to a different oligomer. The shape of the subunit dictates which oligomer is formed. [1] [2] Each oligomer has a finite number of subunits (stoichiometry). Morpheeins can interconvert between forms under physiological conditions and can exist as an equilibrium of different oligomers. These oligomers are physiologically relevant and are not misfolded protein; this distinguishes morpheeins from prions and amyloid. The different oligomers have distinct functionality. Interconversion of morpheein forms can be a structural basis for allosteric regulation. [1] [2] [3] [4] A mutation that shifts the normal equilibrium of morpheein forms can serve as the basis for a conformational disease. [5] Features of morpheeins can be exploited for drug discovery. [1] [3] [6] The dice image (Fig 1) represents a morpheein equilibrium containing two different monomeric shapes that dictate assembly to a tetramer or a pentamer. The one protein that is established to function as a morpheein is porphobilinogen synthase, [2] [7] [8] though there are suggestions throughout the literature that other proteins may function as morpheeins (for more information see "Table of Putative Morpheeins" below).
Implications for drug discovery[edit]
Conformational differences between subunits of different oligomers and related functional differences of a morpheein provide a starting point for drug discovery. Protein function is dependent on the oligomeric form; therefore, the protein’s function can be regulated by shifting the equilibrium of forms. A small molecule compound can shift the equilibrium either by blocking or favoring formation of one of the oligomers. The equilibrium can be shifted using a small molecule that has a preferential binding affinity for only one of the alternate morpheein forms. An inhibitor of porphobilinogen synthase with this mechanism of action has been documented [3] .
Implications for allosteric regulation[edit]
The morpheein model of allosteric regulation has similarities to and differences from other models [1] [9] [4]. The concerted model (the Monod, Wyman and Changeux (MWC) model) of allosteric regulation requires all subunits to be in the same conformation or state within an oligomer like the morpheein model [10] [11]. However, neither this model nor the sequential model (Koshland, Nemethy, and Filmer model) takes into account that the protein may dissociate to interconvert between oligomers [10] [11] [12] [13].
Implications for teaching about protein structure-function relationships[edit]
It is generally taught that a given amino acid sequence will have only one physiologically relevant (native) quaternary structure; morpheeins challenge this concept. The morpheein model does not require gross changes in the basic protein fold [1]. The conformational differences that accompany conversion between oligomers may be similar to the protein motions necessary for function of some proteins [14]. The morpheein model highlights the importance of conformational flexibility for protein functionality and offers a potential explanation for proteins showing non-Michaelis-Menten kinetics, hysteresis, and/or protein concentration dependent specific activity [9].
Implications for understanding the structural basis for disease[edit]
The term “conformational disease” generally encompasses mutations that result in misfolded proteins that aggregate, such as Alzheimer’s and Creutzfeldt-Jakob diseases [15]. In light of the discovery of morpheeins, however, this definition could be expanded to include mutations that shift an equilibrium of alternate oligomeric forms of a protein. An example of such a conformational disease is ALAD porphyria, which results from a mutation of porphobilinogen synthase that causes a shift in its morpheein equilibrium [5].
Table of putative morpheeins[edit]
| Protein | Example species | E.C.number | CAS number | Alternate oligomers | Evidence |
|---|---|---|---|---|---|
| Acetyl-CoA carboxylase-1 | Gallus domesticus | EC 6.4.1.2 | 9023-93-2 | inactive dimer, active dimer, larger [16] | Effector molecules impact multimerization [17], Multiple/protein moonlighting functions [16] |
| α-Acetylgalactosaminidase | Bos taurus | EC 4.3.2.2 | 9027-81-0 | inactive monomer, active tetramer [18] | Substrate binding/turnover impacts multimerization [18], Protein concentration dependent specific activity [19], Different assemblies have different activities [19], Conformationally distinct oligomeric forms [19] [18] |
| Adenylosuccinate lyase | Bacillus subtilis | EC 4.3.2.2 | 9027-81-0 | monomer, dimer, trimer, tetramer [20] | Mutations shift the equilibrium of oligomers [21], Oligomer-dependent kinetic parameters [21], Protein concentration dependent molecular weight [21] |
| Aristolochene synthase | Penicillium roqueforti | EC 4.2.3.9 | 94185-89-4 | monomer, higher order [22] | Protein concentration dependent specific activity [23] |
| L-Asparaginase | Leptosphaeria michotii | EC 3.5.1.1 | 9015-68-3 | dimer, tetramer, inactive octamer [24] | Substrate binding/turnover impacts multimerization [25] |
| Aspartokinase | Escherichia coli | EC 2.7.2.4 & EC 1.1.1.3 | 9012-50-4 | monomer, dimer, tetramer [26] [27] | Multiple/protein moonlighting functions [28], Conformationally distinct oligomeric forms [27] |
| ATPase of the ABCA1 transporter | Homo sapiens | dimer, tetramer [29] | Substrate binding/turnover impacts multimerization [29] | ||
| Biotin—(acetyl-CoA-carboxylase) ligase holoenzyme synthetase | Escherichia coli | EC 6.3.4.15 | 37340-95-7 | monomer, dimer [30] | Multiple/protein moonlighting functions [30], Different assemblies have different activites [31] |
| Chorismate mutase | Escherichia coli | EC 5.4.99.5 | 9068-30-8 | dimer, trimer, hexamer | Conformationally distinct oligomeric forms [32] |
| Citrate synthase | Escherichia coli | EC 2.3.3.1 | 9027-96-7 | monomer, dimer, trimer, tetramer, pentamer, hexamer, dodecamer [33] | Substrate binding/turnover impacts multimerization [33], Characterized equilibrium of oligomers [33], Protein concentration dependent specific activity [33], pH-dependent oligomeric equilibrium [33] |
| Cyanovirin-N | Nostoc ellipsosporum | 918555-82-5 | monomer and domain-swapped dimer [34] [35] | Characterized equilibrium of oligomers [36] [37], Conformationally distinct oligomeric forms [36] [37] | |
| 3-oxoacid CoA-transferase | Sus scrofa domestica | EC 2.8.3.5 | 9027-43-4 | dimer, tetramer [38] | Chromatographically separable oligomers [38], Substrate might preferentially stabilize one form [38] |
| Cystathionine beta-synthase | Homo sapiens | EC 4.2.1.22 | 9023-99-8 | multiple - ranges from dimer to 16-mer [39] | Effector molecules impact multimerization [40], Mutations shift the equilibrium of oligomers [41], Different assemblies have different activities [40], disease-causing mutations at sites distant from active site [42] |
| D-amino acid oxidase | EC 1.4.3.3 | 9000-88-8 | monomers, dimers, higher-order oligomers [43] [44] | Oligomer-dependent kinetic parameters [43] [44] | |
| Dihydrolipoamide dehydrogenase | Sus scrofa domestica | EC 1.8.1.4 | 9001-18-7 | monomer, two different dimer forms, tetramer [45] | Multiple/protein moonlighting functions [45], Different assemblies have different activities [45], pH-dependent oligomeric equilibrium [45], Conformationally distinct oligomeric forms [46] [47] [48] |
| Dopamine beta-monooxygenase | Bos Taurus | EC 1.14.17.1 | 9013-38-1 | dimers, tetramers [49] [50] [51] | Effector molecules impact multimerization [49] [50] [51], Characterized equilibrium of oligomers [49] [50] [51], Oligomer-dependent kinetic parameters [49] [50] [51] |
| Geranylgeranyl pyrophosphate synthase / Farnesyltranstransferase | Homo sapiens | EC 2.5.1.29 | 9032-58-0 | hexamer, octamer [52] [53] [54] | Effector molecules impact multimerization [53] |
| GDP-mannose 6-dehydrogenase | Pseudomonas aeruginosa | EC 1.1.1.132 | 37250-63-8 | trimer, 2 tetramers, and hexamer [55] [56] | Protein concentration dependent specific activity [57], Kinetic hysteresis [57] |
| Glutamate dehydrogenase | Bos taurus | EC 1.4.1.2 | 9001-46-1 | active & inactive hexamers, higher order [58] | Effector molecules impact multimerization [59], Characterized equilibrium of oligomers [58] |
| Glutamate racemase | Mycobacterium tuberculosis, Escherichia coli, Bacillus subtilis, Aquifex pyrophilus | EC 5.1.1.3 | 9024-08-02 | monomer, 2 dimers, tetramer [60] [61] [62] [63] [64] | Multiple/protein moonlighting functions [65] [66] [67], Characterized equilibrium of oligomers [63] [64], Conformationally distinct oligomeric forms [60] [61] [62] |
| Glyceraldehyde-3-phosphate dehydrogenase | Oryctolagus cuniculas, Sus scrofa domestica | EC 1.2.1.12 | 9001-50-7 | monomer, dimer, tetramer [68] | Multiple/protein moonlighting functions [69], Characterized equilibrium of oligomers [68], Different assemblies have different activities [70] |
| Glycerol kinase | Escherichia coli | EC 2.7.1.30 | 9030-66-4 | monomer and 2 tetramers [71] [72] [73] | Characterized equilibrium of oligomers [71] [72] [73] [74], Conformationally distinct oligomeric forms [75] [74], Effector functions by preventing domain motion [75] |
| HIV-Integrase | Human immunodeficiency virus-1 | EC 2.7.7.- | monomer, dimer, tetramer, higher order [76] [77] [78] | Effector molecules impact multimerization [79], Multiple/protein moonlighting functions [76] [77] [78], Different assemblies have different activities [78] [79] | |
| HPr-Kinase/phosphatase | Bacillus subtilis, Lactobacillus casei, Mycoplasma pneumoniae, Staphylococcus xylosus | EC 2.7.1.-/EC 3.1.3.- | 9026-43-1 | monomers, dimers, trimers, hexamers [80] [81] [82] [83] [84] [85] | Effector molecules impact multimerization [84], Multiple/protein moonlighting functions [84], Different assemblies have different activities [84], pH-dependent oligomeric equilibrium [84] |
| Lactate dehydrogenase | Bacillus stearothermophilus | EC 1.1.1.27 | 9001-60-9 | 2 dimers, tetramer [86] [87] | Effector molecules impact multimerization [86] [87], Characterized equilibrium of oligomers [86] [87], Protein concentration dependent specific activity [86] [87], Mutations shift the equilibrium of oligomers [88], Oligomer-dependent kinetic parameters [86] [87], Conformationally distinct oligomeric forms [89] |
| Lon protease | Escherichia coli, Mycobacterium smematis | EC 3.4.21.53 | 79818-35-2 | monomer, dimer, trimer, tetramer [90] [91] | Effector molecules impact multimerization [90] [91], Substrate binding/turnover impacts multimerization [90] [91], Protein concentration dependent specific activity [92], Kinetic hysteresis [92] |
| Mitochondrial NAD(P)+ Malic enzyme / malate dehydrogenase (oxaloacetate-decarboxylating) (NADP+) | Homo sapiens | EC 1.1.1.40 | 9028-47-1 | monomer, 2 dimers, tetramer [93] [94] | Effector molecules impact multimerization [93], Mutations shift the equilibrium of oligomers [95], Kinetic hysteresis [94], |
| Peroxiredoxins | Salmonella typhimurium | EC 1.6.4.- & EC 1.11.1.15 | 207137-51-7 | 2 dimers, decamer [92] | Conformationally distinct oligomeric forms [96], Different assemblies have different activities [97] |
| Phenylalanine hydroxylase | Homo sapiens | EC 1.14.16.1 | 9029-73-6 | high activity tetramer, low activity tetramer [98] | Substrate binding/turnover impacts multimerization [99] [100], Conformationally distinct oligomeric forms [101] [102] |
| Phosphoenolpyruvate carboxylase | Escherichia coli, Zea mays | EC 4.1.1.31 | 9067-77-0 | inactive dimer, active tetramer [103] | Effector molecules impact multimerization, Characterized equilibrium of oligomers [103], Kinetic hysteresis [103], Conformationally distinct oligomeric forms [104] |
| Phosphofructokinase | Bacillus stearothermophilus, Thermus thermophilus | EC 2.7.1.11 | 9001-80-3 | inactive dimer, active tetramer [103] [105] | Effector molecules impact multimerization [103] [105], Characterized equilibrium of oligomers [103] [105] |
| Polyphenol oxidase | Agaricus bisporus, Malus domestica, Lactuca sativa L. | EC 1.10.3.1 | 9002-10-2 | monomer, trimer, tetramer, octamer, dodecamer [106] [107] | Multiple/protein moonlighting functions [108], Substrate binding/turnover impacts multimerization [109], Different assemblies have different activities [110], Kinetic hysteresis [109] |
| Purine nucleoside phosphorylase | Bos Taurus, Escherichia coli | EC 2.4.2.1 | 9030-21-1 | trimer, 2 hexamers [111] [112] [113] | Oligomer-dependent kinetic parameters [111] [114] [115] |
| Pyruvate kinase | Homo sapiens | EC 2.7.1.40 | 9001-59-6 | active and inactive dimers, active tetramer, monomer, trimer, pentamer [116] [117] | Conformationally distinct oligomeric forms [116] [117] |
| Ribonuclease A | Bos taurus | EC 3.1.27.5 | 9901-99-4 | monomer, dimer, trimer, tetramer, hexamer, pentamer, higher order [118] [119] [120] [121] [122] | Multiple/protein moonlighting functions [123] [124] [125], Different assemblies have different activities [123] [124] [125], Conformationally distinct oligomeric forms [119] [121] [122] |
| Ribonucleotide reductase | Mus musculus | EC 1.17.4.1 | 9047-64-7 | tetramer, hexamer [126] [127] [128] [129] | Effector molecules impact multimerization [129] |
| S-adenosyl-L-homocysteine hydrolase | Dictyostelium discoideum | EC 3.3.1.1 | 9025-54-1 | tetramer and other [130] [131] [132] | Effector molecules impact multimerization [130] |
| Biodegrative threonine dehydratase / threonine ammonia-lyase | Escherichia coli | EC 4.3.1.19 | 774231-81-1 | 2 monomers, 2 tetramers [133] [134] [135] | Effector molecules impact multimerization [135], Characterized equilibrium of oligomers [133] [134], Different assemblies have different activities [133] [134] [135] |
| β-Tryptase | Homo sapiens | EC 3.4.21.59 | 97501-93-4 | active and inactive monomers, active and inactive tetramers [136] [137] [138] [139] [140] [141] [142] [143] [144] [145] | Protein concentration dependent specific activity [146], Characterized equilibrium of oligomers [146] |
| Tumor necrosis factor-α | Homo sapiens | 94948-61-5 | monomer, dimer, trimer [147] [148] | Different assemblies have different activities [149] | |
| Uracil phosphoribosyltransferase | Escherichia coli | EC 2.4.2.9 | 9030-24-4 | trimer, pentamer [150] | Effector molecules impact multimerization [150], Substrate binding/turnover impacts multimerization [150], Different assemblies have different activities [150] |
References[edit]
- ^ a b c d e E.K. Jaffe (2005). "Morpheeins - a new structural paradigm for allosteric regulation". Trends Biochem. Sci. 30 (9): 490–497. PMID 16023348. Cite error: The named reference "pmid16023348" was defined multiple times with different content (see the help page).
- ^ a b c S. Breinig, J. Kervinen, L. Stith, A.S. Wasson, R. Fairman, A. Wlodawer, A. Zdanov and E.K. Jaffe (2003). "Control of tetrapyrrole biosynthesis by alternate quaternary forms of porphobilinogen synthase". Nat. Struct. Biol. 10 (9): 757–763. PMID 12897770.
{{cite journal}}: CS1 maint: multiple names: authors list (link) Cite error: The named reference "pmid12897770" was defined multiple times with different content (see the help page). - ^ a b c S.H. Lawrence, U.D. Ramirez, L. Tang, F. Fazliyez, L. Kundrat, G.D. Markham and E.K. Jaffe (2008). "Shape Shifting Leads to Small-Molecule Allosteric Drug Discovery". Chem. Biol. 15 (6): 586–596. PMID 18559269.
{{cite journal}}: CS1 maint: multiple names: authors list (link) Cite error: The named reference "pmid18559269" was defined multiple times with different content (see the help page). - ^ a b c T. Selwood and E. K. Jaffe. (2011). "Dynamic dissociating homo-oligomers and the control of protein function". Arch. Biochem. Biophys. PMID 22182754.
- ^ a b L. Stith and E.K. Jaffe (2007). "ALAD Porphyria Is a Conformational Disease". Am J Hum Genet. 80 (2): 329–337. PMID 17236137. Cite error: The named reference "pmid17236137" was defined multiple times with different content (see the help page).
- ^ E. K. Jaffe (2010). "Morpheeins - A new pathway for allosteric drug discovery". Open Conf. Proc. J. 1: 1–6. PMID 21643557.
- ^ L. Tang, L. Stith and E.K. Jaffe (2005). "Substrate-induced interconversion of protein quaternary structure isoforms". J. Biol. Chem. 280 (16): 15786–15793. PMID 15710608.
- ^ S. H. Lawrence and E. K. Jaffe (2012). "Allostery and the dynamic oligomerization of porphobilinogen synthase". Arch. Biochem. Biophys. PMID 22037356.
- ^ a b S.H. Lawrence and E.K. Jaffe (2008). "Expanding the concepts in protein structure-function relationships and enzyme kinetics: Teaching using morpheeins". Biochemistry and Molecular Biology Education. 36 (4): 274–283. PMID 19578473. Cite error: The named reference "pmid19578473" was defined multiple times with different content (see the help page).
- ^ a b J. Monod, J.P. Changeux and F. Jacob (1963). "Allosteric proteins and cellular control systems". J Mol Biol. 6: 306–29. PMID 13936070. Cite error: The named reference "pmid13936070" was defined multiple times with different content (see the help page).
- ^ a b J. Monod, J. Wyman and J.P. Changeux (1965). "On the nature of allosteric transitions: A plausible model". J. Mol. Biol. 12: 88–118. PMID 14343300. Cite error: The named reference "pmid14343300" was defined multiple times with different content (see the help page).
- ^ D.E. Koshland, Jr (1970). "The molecular basis for enzyme regulation". 1: 341–396.
{{cite journal}}: Cite journal requires|journal=(help) - ^ D.E. Koshland, Jr., G. Nemethy and D. Filmer (1966). "Comparison of experimental binding data and theoretical models in proteins containing subunits". Biochemistry. 5 (1): 365–85. PMID 5938952.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ M. Gerstein and N. Echols (2004). "Exploring the range of protein flexibility, from a structural proteomics perspective". Curr. Opin. Chem. Biol. 8 (1): 14–9. PMID 15036151.
- ^ R.W. Carrell and D.A. Lomas (1997). "Conformational disease". Lancet. 350 (9071): 134–8. PMID 9228977.
- ^ a b R. W. Brownsey, A. N. Boone, J. E. Elliott, J. E. Kulpa and W. M. Lee (2006). "Regulation of acetyl-CoA carboxylase". Biochem Soc Trans. 34: 223–7. PMID 16545081.
{{cite journal}}: CS1 maint: multiple names: authors list (link) Cite error: The named reference "pmid16545081" was defined multiple times with different content (see the help page). - ^ Y. Shen, S>L> Volrath, S.C. Weatherly, T.D. Elich and L. Tong, (2004). "A Mechanism for the Potent Inhibition of Eukaryotic Acetyl-Coenzyme A Carboxylase by Soraphen A, a Macrocyclic Polyketide Natural Product". Molec. Cell. 16 (6): 881–891. PMID 15610732.
{{cite journal}}: CS1 maint: extra punctuation (link) CS1 maint: multiple names: authors list (link) - ^ a b c C. T. Wang and B. Weissmann (1971). "Association-dissociation and abnormal kinetics of bovine alpha-acetylgalactosaminidase". Biochemistry. 10 (6): 1067–72. PMID 5550813. Cite error: The named reference "pmid5550813" was defined multiple times with different content (see the help page).
- ^ a b c B. Weissmann and D.F. Hinrichsen (1969). "Mammalian alpha-acetylgalactosaminidase. Occurrence, partial purification, and action on linkages in submaxillary mucins". Biochemistry. 8 (5): 2034–43. PMID 5785223. Cite error: The named reference "pmid5785223" was defined multiple times with different content (see the help page).
- ^ L. De Zoysa Ariyananda and R. F. Colman (2008). "Evaluation of types of interactions in subunit association in Bacillus subtilis adenylosuccinate lyase". Biochemistry. 47 (9): 2923–34. PMID 18237141.
- ^ a b c J. B. Palenchar and R. F. Colman (2003). "Characterization of a mutant Bacillus subtilis adenylosuccinate lyase equivalent to a mutant enzyme found in human adenylosuccinate lyase deficiency: asparagine 276 plays an important structural role". Biochemistry. 42 (7): 1831–41. PMID 12590570. Cite error: The named reference "pmid12590570" was defined multiple times with different content (see the help page).
- ^ T. M. Hohn and R. D. Plattner (1989). "Purification and characterization of the sesquiterpene cyclase aristolochene synthase from Penicillium roqueforti". Arch Biochem Biophys. 272 (1): 137–43. PMID 2544140.
- ^ J. M. Caruthers, I. Kang, M. J. Rynkiewicz, D. E. Cane and D. W. Christianson (2000). "Crystal structure determination of aristolochene synthase from the blue cheese mold, Penicillium roqueforti". J Biol Chem. 275 (33): 25533–9. PMID 10825154.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ S. Jerebzoff-Quintin and S. Jerebzoff (1985). "L-Asparaginase activity in Leptosphaeria michotii - isolation and properties of 2 forms of the enzyme". Physiol. Plant. 64: 74–80.
- ^ M. K. Yun, A. Nourse, S. W. White, C. O. Rock and R. J. Heath (2007). "Crystal structure and allosteric regulation of the cytoplasmic Escherichia coli L-asparaginase I". J Mol Biol. 369 (3): 794–811. PMID 17451745.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ J. R. Garel and A. Dautry-Varsat (1980). "Sequential folding of a bifunctional allosteric protein". Proc Natl Acad Sci U S A. 77 (6): 3379–83. PMID 6774337.
- ^ a b M. Kotaka, J. Ren, M. Lockyer, A. R. Hawkins, and D. K. Stammers. (2006). "Structures of R- and T-state Escherichia coli aspartokinase III. Mechanisms of the allosteric transition and inhibition by lysine". J. Biol. Chem. 281 (42): 31544–52. PMID 16905770.
{{cite journal}}: CS1 maint: multiple names: authors list (link) Cite error: The named reference "pmid16905770" was defined multiple times with different content (see the help page). - ^ J. W. Ogilvie, L. P. Vickers, R. B. Clark and M. M. Jones (1975). "Aspartokinase I-homoserine dehydrogenase I of Escherichia coli K12 (lambda). Activation by monovalent cations and an analysis of the effect of the adenosine triphosphate-magnesium ion complex on this activation process". J Biol Chem. 250 (4): 1242–50. PMID 163250.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ a b D. Trompier, M. Alibert, S. Davanture, Y. Hamon, M. Pierres and G. Chimini (2006). "Transition from dimers to higher oligomeric forms occurs during the ATPase cycle of the ABCA1 transporter". J Biol Chem. 281 (29): 20283–90. PMID 16709568.
{{cite journal}}: CS1 maint: multiple names: authors list (link) Cite error: The named reference "pmid16709568" was defined multiple times with different content (see the help page). - ^ a b E. Eisenstein and D. Beckett. (1999). "Dimerization of the Escherichia coli biotin repressor: corepressor function in protein assembly". Biochemistry. 38 (40): 13077–84. PMID 10529178. Cite error: The named reference "pmid10529178" was defined multiple times with different content (see the help page).
- ^ E.D. Streaker and D. Beckett. (1998). "Coupling of site-specific DNA binding to protein dimerization in assembly of the biotin repressor-biotin operator complex". Biochemistry. 37 (9): 3210–9. PMID 9485476.
- ^ K. Vamvaca, M. Butz, K.U. Walter, S.V. Taylor and D. Hilvert. (2005). "Simultaneous optimization of enzyme activity and quaternary structure by directed evolution". Protein Sci. 14 (8): 2103–14. PMID 15987889.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ a b c d e E.K. Tong and H.W. Duckworth (1975). "The quaternary structure of citrate synthase from Escherichia coli K12". Biochemistry. 14 (2): 235–41. PMID 1091285. Cite error: The named reference "pmid1091285" was defined multiple times with different content (see the help page).
- ^ C. A. Bewley, K. R. Gustafson, M. R. Boyd, D. G. Covell, A. Bax, G. M. Clore and A. M. Gronenborn (1998). "Solution structure of cyanovirin-N, a potent HIV-inactivating protein". Nat Struct Biol. 5 (7): 571–8. PMID 9665171.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ F. Yang, C. A. Bewley, J. M. Louis, K. R. Gustafson, M. R. Boyd, A. M. Gronenborn, G. M. Clore and A. Wlodawer (1999). "Crystal structure of cyanovirin-N, a potent HIV-inactivating protein, shows unexpected domain swapping". J Mol Biol. 288 (3): 403–12. PMID 10329150.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ a b L. G. Barrientos and A. M. Gronenborn (2005). "The highly specific carbohydrate-binding protein cyanovirin-N: structure, anti-HIV/Ebola activity and possibilities for therapy". Mini Rev Med Chem. 5 (1): 21–31. PMID 15638789. Cite error: The named reference "pmid15638789" was defined multiple times with different content (see the help page).
- ^ a b L. G. Barrientos, J. M. Louis, I. Botos, T. Mori, Z. Han, B. R. O'Keefe, M. R. Boyd, A. Wlodawer and A. M. Gronenborn (2002). "The domain-swapped dimer of cyanovirin-N is in a metastable folded state: reconciliation of X-ray and NMR structures". Structure. 10 (5): 673–86. PMID 12015150.
{{cite journal}}: CS1 maint: multiple names: authors list (link) Cite error: The named reference "pmid12015150" was defined multiple times with different content (see the help page). - ^ a b c J. C. Rochet, E. R. Brownie, K. Oikawa, L. D. Hicks, M. E. Fraser, M. N. James, C. M. Kay, W. A. Bridger and W. T. Wolodko (2000). "Pig heart CoA transferase exists as two oligomeric forms separated by a large kinetic barrier". Biochemistry. 39 (37): 11291–302. PMID 10985774.
{{cite journal}}: CS1 maint: multiple names: authors list (link) Cite error: The named reference "pmid10985774" was defined multiple times with different content (see the help page). - ^ N. Frank, V. Kery, K. N. Maclean and J. P. Kraus (2006). "Solvent-accessible cysteines in human cystathionine beta-synthase: crucial role of cysteine 431 in S-adenosyl-L-methionine binding". Biochemistry. 45 (36): 11021–9. PMID 16953589.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ a b S. Sen and R. Banerjee (2007). "A pathogenic linked mutation in the catalytic core of human cystathionine beta-synthase disrupts allosteric regulation and allows kinetic characterization of a full-length dimer". Biochemistry. 46 (13): 4110–6. PMID 17352495. Cite error: The named reference "pmid17352495" was defined multiple times with different content (see the help page).
- ^ V. Kery, L. Poneleit and J. P. Kraus (1998). "Trypsin cleavage of human cystathionine beta-synthase into an evolutionarily conserved active core: structural and functional consequences". Arch Biochem Biophys. 355 (2): 222–32. PMID 9675031.
- ^ X. Shan and W. D. Kruger (1998). "Correction of disease-causing CBS mutations in yeast". Nat Genet. 19 (1): 91–3. PMID 9590298.
- ^ a b E. Antonini, M. Brunori, R. Bruzzesi, E. Chiancone and V. Massey (1966). "Association-dissociation phenomena of D-amino acid oxidase". J Biol Chem. 241 (10): 2358–66. PMID 4380380.
{{cite journal}}: CS1 maint: multiple names: authors list (link) Cite error: The named reference "pmid4380380" was defined multiple times with different content (see the help page). - ^ a b V. Massey, B. Curti and H. Ganther (1966). "A temperature-dependent conformational change in D-amino acid oxidase and its effect on catalysis". J Biol Chem. 241 (10): 2347–57. PMID 5911617. Cite error: The named reference "pmid5911617" was defined multiple times with different content (see the help page).
- ^ a b c d N. E. Babady, Y. P. Pang, O. Elpeleg and G. Isaya (2007). "Cryptic proteolytic activity of dihydrolipoamide dehydrogenase". Proc Natl Acad Sci U S A. 104 (15): 6158–63. PMID 17404228.
{{cite journal}}: CS1 maint: multiple names: authors list (link) Cite error: The named reference "pmid17404228" was defined multiple times with different content (see the help page). - ^ H. v Muiswinkel-Voetberg, J. Visser and C. Veeger (1973). "Conformational studies on lipoamide dehydrogenase from pig heart. I. Interconversion of dissociable and non-dissociable forms". Eur J Biochem. 33 (2): 265–70. PMID 4348439.
- ^ N. L. Klyachko, V. A. Shchedrina, A. V. Efimov, S. V. Kazakov, I. G. Gazaryan, B. S. Kristal and A. M. Brown (2005). "pH-dependent substrate preference of pig heart lipoamide dehydrogenase varies with oligomeric state: response to mitochondrial matrix acidification". J Biol Chem. 280 (16): 16106–14. PMID 15710613.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ H. v Muiswinkel-Voetberg and C. Veeger (1973). "Conformational studies on lipoamide dehydrogenase from pig heart. 2. Spectroscopic studies on the apoenzyme and the monomeric and dimeric forms". Eur J Biochem. 33 (2): 271–8. PMID 4348440.
- ^ a b c d A. Saxena, P. Hensley, J.C. Osborne Jr. and P.J. Fleming. (1985). "The pH-dependent subunit dissociation and catalytic activity of bovine dopamine beta-hydroxylase". J. Biol. Chem. 260 (6): 3386–92. PMID 3972830.
{{cite journal}}: CS1 maint: multiple names: authors list (link) Cite error: The named reference "pmid3972830" was defined multiple times with different content (see the help page). - ^ a b c d S. Dhawan, P. Hensley, J.C. Osborne Jr. and P.J. Fleming. (1986). "Adenosine 5'-diphosphate-dependent subunit dissociation of bovine dopamine beta-hydroxylase". J. Biol. Chem. 261 (17): 7680–4. PMID 3711102.
{{cite journal}}: CS1 maint: multiple names: authors list (link) Cite error: The named reference "pmid3711102" was defined multiple times with different content (see the help page). - ^ a b c d L.C. Stewart and J.P. Klinman. (1988). "Dopamine beta-hydroxylase of adrenal chromaffin granules: structure and function". Annu. Rev. Biochem. 57: 551–92. PMID 3052283. Cite error: The named reference "pmid3052283" was defined multiple times with different content (see the help page).
- ^ T. Kuzuguchi, Y. Morita, I. Sagami, H. Sagami and K. Ogura. (1999). "Human geranylgeranyl diphosphate synthase: cDNA cloning and expression". J. Biol. Chem. 274 (9): 5888–94. PMID 10026212.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ a b K.L. Kavanagh, J.E. Dunford, G. Bunkoczi, R.G. Russell and U. Oppermann. (2006). "The crystal structure of human geranylgeranyl pyrophosphate synthase reveals a novel hexameric arrangement and inhibitory product binding". J. Biol. Chem. 281 (31): 22004–12. PMID 16698791.
{{cite journal}}: CS1 maint: multiple names: authors list (link) Cite error: The named reference "pmid16698791" was defined multiple times with different content (see the help page). - ^ Y. Myagi, Y. Matsumura, and H. Sagami. (2007). "Human geranylgeranyl diphosphate synthase is an octamer in solution". J. Biochem. 142 (3): 377–81. PMID 17646172.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ C.F. Snook, P.A. Tipton, and L.J. Beamer. (2003). "Crystal structure of GDP-mannose dehydrogenase: a key enzyme of alginate biosynthesis in P. aeruginosa". Biochemistry. 42 (16): 4658–668. PMID 12705829.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ S. Roychoudhury, T. B. May, J. F. Gill, S. K. Singh, D. S. Feingold and A. M. Chakrabarty. (1989). "Purification and characterization of guanosine diphospho-D-mannose dehydrogenase. A key enzyme in the biosynthesis of alginate by Pseudomonas aeruginosa". J. Biol. Chem. 264 (16): 9380–5. PMID 2470755.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ a b L. E. Naught, S. Gilbert, R. Imhoff, C. Snook, L. Beamer and P. Tipton. (2002). "Allosterism and cooperativity in Pseudomonas aeruginosa GDP-mannose dehydrogenase". Biochemistry. 41 (30): 9637–45. PMID 12135385.
{{cite journal}}: CS1 maint: multiple names: authors list (link) Cite error: The named reference "pmid12135385" was defined multiple times with different content (see the help page). - ^ a b H. F. Fisher (1973). "Glutamate dehydrogenase--ligand complexes and their relationship to the mechanism of the reaction". Adv Enzymol Relat Areas Mol Biol. 39 (40): 369–417. PMID 4147773. Cite error: The named reference "pmid4147773" was defined multiple times with different content (see the help page).
- ^ C. Y. Huang and C. Frieden (1972). "The mechanism of ligand-induced structural changes in glutamate dehydrogenase. Studies of the rate of depolymerization and isomerization effected by coenzymes and guanine nucleotides". J Biol Chem. 247 (11): 3638–46. PMID 4402280.
- ^ a b S. S. Kim, I. G. Choi, S. H. Kim and Y. G. Yu (1999). "Molecular cloning, expression, and characterization of a thermostable glutamate racemase from a hyperthermophilic bacterium, Aquifex pyrophilus". Extremophiles. 3 (3): 175–83. PMID 10484173.
{{cite journal}}: CS1 maint: multiple names: authors list (link) Cite error: The named reference "pmid10484173" was defined multiple times with different content (see the help page). - ^ a b T. Lundqvist, S. L. Fisher, G. Kern, R. H. Folmer, Y. Xue, D. T. Newton, T. A. Keating, R. A. Alm and B. L. de Jonge (2007). "Exploitation of structural and regulatory diversity in glutamate racemases". Nature. 447 (7146): 817–22. PMID 17568739.
{{cite journal}}: CS1 maint: multiple names: authors list (link) Cite error: The named reference "pmid17568739" was defined multiple times with different content (see the help page). - ^ a b M. May, S. Mehboob, D. C. Mulhearn, Z. Wang, H. Yu, G. R. Thatcher, B. D. Santarsiero, M. E. Johnson and A. D. Mesecar (2007). "Structural and functional analysis of two glutamate racemase isozymes from Bacillus anthracis and implications for inhibitor design". J Mol Biol. 371 (5): 1219–37. PMID 17610893.
{{cite journal}}: CS1 maint: multiple names: authors list (link) Cite error: The named reference "pmid17610893" was defined multiple times with different content (see the help page). - ^ a b M. A. Taal, S. E. Sedelnikova, S. N. Ruzheinikov, P. J. Baker and D. W. Rice (2004). "Expression, purification and preliminary X-ray analysis of crystals of Bacillus subtilis glutamate racemase". Acta Crystallogr D Biol Crystallogr. 60 (16): 2031–4. PMID 15502318.
{{cite journal}}: CS1 maint: multiple names: authors list (link) Cite error: The named reference "pmid15502318" was defined multiple times with different content (see the help page). - ^ a b K. H. Kim, Y. J. Bong, J. K. Park, K. J. Shin, K. Y. Hwang and E. E. Kim (2007). "Structural basis for glutamate racemase inhibition". J Mol Biol. 372 (2): 434–43. PMID 17658548.
{{cite journal}}: CS1 maint: multiple names: authors list (link) Cite error: The named reference "pmid17658548" was defined multiple times with different content (see the help page). - ^ M. Ashiuchi, E. Kuwana, T. Yamamoto, K. Komatsu, K. Soda and H. Misono (2002). "Glutamate racemase is an endogenous DNA gyrase inhibitor". J Biol Chem. 277 (42): 39070–3. PMID 12213801.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ M. Ashiuchi, K. Tani, K. Soda and H. Misono (1998). "Properties of glutamate racemase from Bacillus subtilis IFO 3336 producing poly-gamma-glutamate". J Biochem. 123 (6): 1156–63. PMID 9604005.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ S. Sengupta, S. Ghosh and V. Nagaraja (2008). "Moonlighting function of glutamate racemase from Mycobacterium tuberculosis: racemization and DNA gyrase inhibition are two independent activities of the enzyme". Microbiology. 154: 2796–803. PMID 18757813.
- ^ a b S. M. Constantinides and W. C. Deal, Jr. (1969). "Reversible dissociation of tetrameric rabbit muscle glyceraldehyde 3-phosphate dehydrogenase into dimers or monomers by adenosine triphosphate". J Biol Chem. 244 (20): 5695–702. PMID 4312250. Cite error: The named reference "pmid4312250" was defined multiple times with different content (see the help page).
- ^ M. A. Sirover (1999). "New insights into an old protein: the functional diversity of mammalian glyceraldehyde-3-phosphate dehydrogenase". Biochim Biophys Acta. 1432 (2): 159–84. PMID 10407139.
- ^ H. Kumagai and H. Sakai (1983). "A porcine brain protein (35 K protein) which bundles microtubules and its identification as glyceraldehyde 3-phosphate dehydrogenase". J Biochem (Tokyo). 93 (5): 1259–69. PMID 6885722.
- ^ a b J. K. de Riel and H. Paulus (1978). "Subunit dissociation in the allosteric regulation of glycerol kinase from Escherichia coli. 2. Physical evidence". Biochemistry. 17 (24): 5141–6. PMID 215195. Cite error: The named reference "pmid215195" was defined multiple times with different content (see the help page).
- ^ a b J. K. de Riel and H. Paulus (1978). "Subunit dissociation in the allosteric regulation of glycerol kinase from Escherichia coli. 1. Kinetic evidence". Biochemistry. 17 (24): 5134–40. PMID 215194. Cite error: The named reference "pmid215194" was defined multiple times with different content (see the help page).
- ^ a b J. K. de Riel and H. Paulus (1978). "Subunit dissociation in the allosteric regulation of Glycerol kinase from Escherichia coli. 3. Role in desensitization". Biochemistry. 17 (24): 5146–50. PMID 31903. Cite error: The named reference "pmid31903" was defined multiple times with different content (see the help page).
- ^ a b M. D. Feese, H. R. Faber, C. E. Bystrom, D. W. Pettigrew and S. J. Remington (1998). "Glycerol kinase from Escherichia coli and an Ala65-->Thr mutant: the crystal structures reveal conformational changes with implications for allosteric regulation". Structure. 6 (11): 1407–18. PMID 9817843.
{{cite journal}}: CS1 maint: multiple names: authors list (link) Cite error: The named reference "pmid9817843" was defined multiple times with different content (see the help page). - ^ a b C. E. Bystrom, D. W. Pettigrew, B. P. Branchaud, P. O'Brien and S. J. Remington (1999). "Crystal structures of Escherichia coli glycerol kinase variant S58-->W in complex with nonhydrolyzable ATP analogues reveal a putative active conformation of the enzyme as a result of domain motion". Biochemistry. 38 (12): 3508–18. PMID 10090737.
{{cite journal}}: CS1 maint: multiple names: authors list (link) Cite error: The named reference "pmid10090737" was defined multiple times with different content (see the help page). - ^ a b E. Deprez, P. Tauc, H. Leh, J. F. Mouscadet, C. Auclair and J. C. Brochon (2000). "Oligomeric states of the HIV-1 integrase as measured by time-resolved fluorescence anisotropy". Biochemistry. 39 (31): 9275–84. PMID 10924120.
{{cite journal}}: CS1 maint: multiple names: authors list (link) Cite error: The named reference "pmid10924120" was defined multiple times with different content (see the help page). - ^ a b E. Deprez, P. Tauc, H. Leh, J. F. Mouscadet, C. Auclair, M. E. Hawkins and J. C. Brochon (2001). "DNA binding induces dissociation of the multimeric form of HIV-1 integrase: a time-resolved fluorescence anisotropy study". Proc Natl Acad Sci U S A. 98 (18): 10090–5. PMID 11504911.
{{cite journal}}: CS1 maint: multiple names: authors list (link) Cite error: The named reference "pmid11504911" was defined multiple times with different content (see the help page). - ^ a b c A. Faure, C. Calmels, C. Desjobert, M. Castroviejo, A. Caumont-Sarcos, L. Tarrago-Litvak, S. Litvak and V. Parissi (2005). "HIV-1 integrase crosslinked oligomers are active in vitro". Nucleic Acids Res. 33 (3): 977–86. PMID 15718297.
{{cite journal}}: CS1 maint: multiple names: authors list (link) Cite error: The named reference "pmid15718297" was defined multiple times with different content (see the help page). - ^ a b E. Guiot, K. Carayon, O. Delelis, F. Simon, P. Tauc, E. Zubin, M. Gottikh, J. F. Mouscadet, J. C. Brochon and E. Deprez. (2006). "Relationship between the oligomeric status of HIV-1 integrase on DNA and enzymatic activity". J. Biol. Chem. 281 (32): 22707–19. PMID 16774912.
{{cite journal}}: CS1 maint: multiple names: authors list (link) Cite error: The named reference "pmid16774912" was defined multiple times with different content (see the help page). - ^ S. Fieulaine, S. Morera, S. Poncet, V. Monedero, V. Gueguen-Chaignon, A. Galinier, J. Janin, J. Deutscher and S. Nessler (2001). "X-ray structure of HPr kinase: a bacterial protein kinase with a P-loop nucleotide-binding domain". EMBO J. 20 (15): 3917–27. PMID 11483495.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ J. A. Marquez, S. Hasenbein, B. Koch, S. Fieulaine, S. Nessler, R. B. Russell, W. Hengstenberg and K. Scheffzek (2002). "Structure of the full-length HPr kinase/phosphatase from Staphylococcus xylosus at 1.95 A resolution: Mimicking the product/substrate of the phospho transfer reactions". Proc Natl Acad Sci U S A. 99 (6): 3458–63. PMID 11904409.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ G. S. Allen, K. Steinhauer, W. Hillen, J. Stulke and R. G. Brennan (2003). "Crystal structure of HPr kinase/phosphatase from Mycoplasma pneumoniae". J Mol Biol. 326 (4): 1203–17. PMID 12589763.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ S. Poncet, I. Mijakovic, S. Nessler, V. Gueguen-Chaignon, V. Chaptal, A. Galinier, G. Boel, A. Maze and J. Deutscher (2004). "HPr kinase/phosphorylase, a Walker motif A-containing bifunctional sensor enzyme controlling catabolite repression in Gram-positive bacteria". Biochim Biophys Acta. 1697 (1): 123–35. PMID 15023355.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ a b c d e H. Ramstrom, S. Sanglier, E. Leize-Wagner, C. Philippe, A. Van Dorsselaer and J. Haiech (2003). "Properties and regulation of the bifunctional enzyme HPr kinase/phosphatase in Bacillus subtilis". J Biol Chem. 278 (2): 1174–85. PMID 12411438.
{{cite journal}}: CS1 maint: multiple names: authors list (link) Cite error: The named reference "pmid12411438" was defined multiple times with different content (see the help page). - ^ J. M. Jault, S. Fieulaine, S. Nessler, P. Gonzalo, A. Di Pietro, J. Deutscher and A. Galinier (2000). "The HPr kinase from Bacillus subtilis is a homo-oligomeric enzyme which exhibits strong positive cooperativity for nucleotide and fructose 1,6-bisphosphate binding". J Biol Chem. 275 (3): 1773–80. PMID 10636874.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ a b c d e A.R. Clarke, A.D. Waldman, K.W. Hart and J.J. Holbrook. (1985). "Changes in the state of subunit association of lactate dehydrogenase from Bacillus stearothermophilus". Biochim. Biophys. Acta. 828 (3): 375–9. PMID 3986214.
{{cite journal}}: CS1 maint: multiple names: authors list (link) Cite error: The named reference "pmid3986214" was defined multiple times with different content (see the help page). - ^ a b c d e A.R. Clarke, A.D. Waldman, I. Munro and J.J. Holbrook. (1985). "The rates of defined changes in protein structure during the catalytic cycle of lactate dehydrogenase". Biochim Biophys Acta. 829 (3): 397–407. PMID 4005269.
{{cite journal}}: CS1 maint: multiple names: authors list (link) Cite error: The named reference "pmid4005269" was defined multiple times with different content (see the help page). - ^ A. R. Clarke, D. B. Wigley, D. A. Barstow, W. N. Chia, T. Atkinson and J. J. Holbrook (1987). "A single amino acid substitution deregulates a bacterial lactate dehydrogenase and stabilizes its tetrameric structure". Biochim Biophys Acta. 913 (1): 72–80. PMID 3580377.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ A. D. Cameron, D. I. Roper, K. M. Moreton, H. Muirhead, J. J. Holbrook and D. B. Wigley (1994). "Allosteric activation in Bacillus stearothermophilus lactate dehydrogenase investigated by an X-ray crystallographic analysis of a mutant designed to prevent tetramerization of the enzyme". J Mol Biol. 238 (4): 615–25. PMID 8176749.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ a b c S. G. Roudiak and T. E. Shrader. (1998). "Functional role of the N-terminal region of the Lon protease from Mycobacterium smegmatis". Biochemistry. 37 (32): 11255–63. PMID 9698372. Cite error: The named reference "pmid9698372" was defined multiple times with different content (see the help page).
- ^ a b c S. G. Rudyak, M. Brenowitz and T. E. Shrader. (2001). "Mg2+-linked oligomerization modulates the catalytic activity of the Lon (La) protease from Mycobacterium smegmatis". Biochemistry. 40 (31): 9317–23. PMID 11478899. Cite error: The named reference "pmid11478899" was defined multiple times with different content (see the help page).
- ^ a b c D. Vineyard, J. Patterson-Ward and I. Lee. (2006). "Single-turnover kinetic experiments confirm the existence of high- and low-affinity ATPase sites in Escherichia coli Lon protease". Biochemistry. 45 (14): 4602–10. PMID 16584195. Cite error: The named reference "pmid16584195" was defined multiple times with different content (see the help page).
- ^ a b Z. Yang, C. W. Lanks and L. Tong (2002). "Molecular mechanism for the regulation of human mitochondrial NAD(P)+-dependent malic enzyme by ATP and fumarate". Structure. 10 (7): 951–60. PMID 12121650. Cite error: The named reference "pmid12121650" was defined multiple times with different content (see the help page).
- ^ a b G. E. Edwards and C. S. Andreo (1992). "NADP-malic enzyme from plants". Phytochemistry. 31 (6): 1845–57. PMID 1368216. Cite error: The named reference "pmid1368216" was defined multiple times with different content (see the help page).
- ^ J. Y. Hsieh, S. H. Chen and H. C. Hung (2009). "Functional roles of the tetramer organization of malic enzyme". J Biol Chem. 284 (27): 18096–105. PMID 19416979.
- ^ L. B. Poole (2005). "Bacterial defenses against oxidants: mechanistic features of cysteine-based peroxidases and their flavoprotein reductases". Arch. Biochem. Biophys. 433 (1): 240–254. PMID 15581580.
- ^ M. Aran, D. S. Ferrero, E. Pagano and R. A. Wolosiuk (2009). "Typical 2-Cys peroxiredoxins--modulation by covalent transformations and noncovalent interactions". FEBS J. 276 (9): 2478–93. PMID 19476489.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ E. Bjorgo, R. M. de Carvalho and T. Flatmark. (2001). "A comparison of kinetic and regulatory properties of the tetrameric and dimeric forms of wild-type and Thr427Pro mutant human phenylalanine hydroxylase: contribution of the flexible hinge region Asp425-Gln429 to the tetramerization and cooperative substrate binding". Eur. J. Biochem. 268 (4): 997–1005. PMID 11179966.
- ^ A. Martinez, P. M. Knappskog, S. Olafsdottir, A. P. Doskeland, H. G. Eiken, R. M. Svebak, M. Bozzini, J. Apold and T. Flatmark (1995). "Expression of recombinant human phenylalanine hydroxylase as fusion protein in Escherichia coli circumvents proteolytic degradation by host cell proteases. Isolation and characterization of the wild-type enzyme". Biochem J. 306: 589–97. PMID 7887915.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ P. M. Knappskog, T. Flatmark, J. M. Aarden, J. Haavik and A. Martinez (1996). "Structure/function relationships in human phenylalanine hydroxylase - Effect of terminal deletions on the oligomerization, activation and cooperativity of substrate binding to the enzyme". European Journal of Biochemistry. 242 (3): 813–821. PMID 9022714.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ R. S. Phillips, M. A. Parniak and S. Kaufman (1984). "Spectroscopic investigation of ligand interaction with hepatic phenylalanine hydroxylase: evidence for a conformational change associated with activation". Biochemistry. 23 (17): 3836–42. PMID 6487579.
- ^ F. Fusetti, H. Erlandsen, T. Flatmark and R. C. Stevens (1998). "Structure of tetrameric human phenylalanine hydroxylase and its implications for phenylketonuria". J Biol Chem. 273 (27): 16962–7. PMID 9642259.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ a b c d e f R. C. Wohl and G. Markus (1972). "Phosphoenolpyruvate carboxylase of Escherichia coli. Purification and some properties". J Biol Chem. 247 (18): 5785–92. PMID 4560418. Cite error: The named reference "pmid4560418" was defined multiple times with different content (see the help page).
- ^ Y. Kai, H. Matsumura and K. Izui (2003). "Phosphoenolpyruvate carboxylase: three-dimensional structure and molecular mechanisms". Arch Biochem Biophys. 414 (2): 170–9. PMID 12781768.
- ^ a b c J. Xu, T. Oshima and M. Yoshida (1990). "Tetramer-dimer conversion of phosphofructokinase from Thermus thermophilus induced by its allosteric effectors". J Mol Biol. 215 (4): 597–606. PMID 2146397. Cite error: The named reference "pmid2146397" was defined multiple times with different content (see the help page).
- ^ R. L. Jolley, Jr. and H. S. Mason (1965). "The Multiple Forms of Mushroom Tyrosinase. Interconversion". J Biol Chem. 240. PMID 14284774.
- ^ R. L. Jolley, Jr., D. A. Robb and H. S. Mason (1969). "The multiple forms of mushroom tyrosinase. Association-dissociation phenomena". J Biol Chem. 244 (6): 1593–9. PMID 4975157.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ M.F. Mallette and C.R. Dawson. (1949). "On the nature of highly purified mushroom tyrosinase preparations". Arch. Biochem. 23 (1): 29–44. PMID 18135760.
- ^ a b S. Chazarra, F. Garcia-Carmona and J. Cabanes (2001). "Hysteresis and positive cooperativity of iceberg lettuce polyphenol oxidase". Biochem Biophys Res Commun. 289 (3): 769–75. PMID 11726215.
- ^ E. Harel and A. M. Mayer (1968). "Interconversion of sub-units of catechol oxidase from apple chloroplasts". Phytochemistry. 7 (2): 199–204.
- ^ a b A. Bzowska, E. Kulikowska and D. Shugar (2000). "Purine nucleoside phosphorylases: properties, functions, and clinical aspects". Pharmacol Ther. 88 (3): 349–425. PMID 11337031.
- ^ A. Bzowska, M. Luic, W. Schroder, D. Shugar, W. Saenger and G. Koellner (1995). "Calf spleen purine nucleoside phosphorylase: purification, sequence and crystal structure of its complex with an N(7)-acycloguanosine inhibitor". FEBS Lett. 367 (3): 214–8. PMID 7607309.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ A. Fedorov, W. Shi, G. Kicska, E. Fedorov, P. C. Tyler, R. H. Furneaux, J. C. Hanson, G. J. Gainsford, J. Z. Larese, V. L. Schramm and S. C. Almo (2001). "Transition state structure of purine nucleoside phosphorylase and principles of atomic motion in enzymatic catalysis". Biochemistry. 40 (4): 853–60. PMID 11170405.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ D. J. Porter (1992). "Purine nucleoside phosphorylase. Kinetic mechanism of the enzyme from calf spleen". J Biol Chem. 267 (11): 7342–51. PMID 1559977.
- ^ B. Wielgus-Kutrowska (1999). "Purine nucleoside phosphorylase - physiochemical properties and mechanism of interaction with ligands". University of Warsaw.
- ^ a b J. Schulz, G. Sparmann and E. Hofmann (1975). "Alanine-mediated reversible inactivation of tumour pyruvate kinase caused by a tetramer-dimer transition". FEBS Lett. 50 (3): 346–50. PMID 1116605. Cite error: The named reference "pmid1116605" was defined multiple times with different content (see the help page).
- ^ a b K. H. Ibsen, K. W. Schiller and T. A. Haas (1971). "Interconvertible kinetic and physical forms of human erythrocyte pyruvate kinase". J Biol Chem. 246 (5): 1233–40. PMID 5545066. Cite error: The named reference "pmid5545066" was defined multiple times with different content (see the help page).
- ^ Y. Liu, G. Gotte, M. Libonati and D. Eisenberg (2002). "Structures of the two 3D domain-swapped RNase A trimers". Protein Sci. 11 (2): 371–80. PMID 11790847.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ a b G. Gotte, M. Bertoldi and M. Libonati (1999). "Structural versatility of bovine ribonuclease A - Distinct conformers of trimeric and tetrameric aggregates of the enzyme". European Journal of Biochemistry. 265 (2): 680–687. PMID 10504400.
- ^ G. Gotte, D. V. Laurents and M. Libonati (2006). "Three-dimensional domain-swapped oligomers of ribonuclease A: Identification of a fifth tetramer, pentamers and hexamers, and detection of trace heptameric, octameric and nonameric species". Biochimica Et Biophysica Acta-Proteins and Proteomics. 1764 (1): 44–54. PMID 16310422.
- ^ a b G. Gotte and M. Libonati (1998). [9675255 "Two different forms of aggregated dimers of ribonuclease A"]. Biochimica Et Biophysica Acta-Protein Structure and Molecular Enzymology. 1386 (1): 106–112. PMID http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=9675255.
{{cite journal}}: Check|pmid=value (help); Check|url=value (help); External link in|pmid= - ^ a b M. Libonati and G. Gotte (2004). "Oligomerization of bovine ribonuclease A: structural and functional features of its multimers". Biochem J. 380 (Pt2): 311–327. PMID 15104538.
- ^ a b M. Libonati (2004). "Biological actions of the oligomers of ribonuclease A". Cell Molec Life Sci. 61 (19): 2431–2436. PMID 15526151.
- ^ a b M. Libonati, M. Bertoldi and S. Sorrentino (1996). "The activity on double-stranded RNA of aggregates of ribonuclease A higher than dimers increases as a function of the size of the aggregates". Biochem J. 318 (Pt1): 287–290. PMID 8761484.
- ^ a b M. Libonati, G. Gotte and F. Vottariello (2008). "A novel biological actions acquired by ribonuclease through oligomerization". Curr Pharm Biotech. 9 (3): 200–209. PMID 18673285.
- ^ O. B. Kashlan and B. S. Cooperman (2003). "Comprehensive model for allosteric regulation of mammalian ribonucleotide reductase: refinements and consequences". Biochemistry. 42 (6): 1696–706. PMID 12578384.
- ^ O. B. Kashlan, C. P. Scott, J. D. Lear and B. S. Cooperman (2002). "A comprehensive model for the allosteric regulation of mammalian ribonucleotide reductase. Functional consequences of ATP- and dATP-induced oligomerization of the large subunit". Biochemistry. 41 (2): 462–74. PMID 11781084.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ M. Eriksson, U. Uhlin, S. Ramaswamy, M. Ekberg, K. Regnstrom, B. M. Sjoberg and H. Eklund (1997). "Binding of allosteric effectors to ribonucleotide reductase protein R1: reduction of active-site cysteines promotes substrate binding". Structure. 5 (8): 1077–92. PMID 9309223.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ a b J.W. Fairman, S.R. Wijeranthna, M.F. Ahmad, H. Xu, R. Nakano, S. Jha, J. Prendergast, R.M. Welin, S. Flodin, A. Roos, P. Norlund, Z. Li, T. Walz and C.G. Dealwis. (2011). "Structural basis for allosteric regulation of human ribonucleotide reductase by nucleotide-induced oligomerization". Nat. Struct. Mol. Biol. 18 (3): 316–22. PMID 21336276.
{{cite journal}}: CS1 maint: multiple names: authors list (link) Cite error: The named reference "pmid21336276" was defined multiple times with different content (see the help page). - ^ a b R. J. Hohman, M. C. Guitton and M. Veron (1984). "Purification of S-adenosyl-L-homocysteine hydrolase from Dictyostelium discoideum: reversible inactivation by cAMP and 2'-deoxyadenosine". Arch Biochem Biophys. 233 (2): 785–95. PMID 6091559.
- ^ A. Guranowski and J. Pawelkiewicz (1977). "Adenosylhomocysteinase from yellow lupin seeds. Purification and properties". Eur J Biochem. 80 (2): 517–23. PMID 923592.
- ^ E. O. Kajander and A. M. Raina (1981). "Affinity-chromatographic purification of S-adenosyl-L-homocysteine hydrolase. Some properties of the enzyme from rat liver". Biochem J. 193 (2): 503–12. PMID 7305945.
- ^ a b c Y. Saeki, S. Ito, Y. Shizuta, O. Hayaishi, H. Kagamiyama and H. Wada (1977). "Subunit structure of biodegradative threonine deaminase". J. Biol. Chem. 252 (7): 2206–8. PMID 321452.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ a b c A. T. Phillips and W. A. Wood (1964). "Basis for AMP activation of "Biodegradative" threonine dehydrase from". Biochem Biophys Res Commun. 15 (6): 530–535. PMID 5321308.
- ^ a b c J. A. Gerlt, K. W. Rabinowitz, C. P. Dunne and W. A. Wood (1973). "The mechanism of action of 5'-adenylic acid-activated threonine dehydrase. V. Relation between ligand-induced allosteric activation and the protomeroligomer interconversion". J Biol Chem. 248 (23): 8200–6. PMID 4584826.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ A. K. Addington and D. A. Johnson (1996). "Inactivation of human lung tryptase: evidence for a re-activatable tetrameric intermediate and active monomers". Biochemistry. 35 (42): 13511–8. PMID 8885830.
- ^ I. Fajardo and G. Pejler (2003). "Formation of active monomers from tetrameric human beta-tryptase". Biochem J. 369: 603–10. PMID 12387726.
- ^ Y. Fukuoka and L. B. Schwartz (2004). "Human beta-tryptase: detection and characterization of the active monomer and prevention of tetramer reconstitution by protease inhibitors". Biochemistry. 43 (33): 10757–64. PMID 15311937.
- ^ Y. Fukuoka and L. B. Schwartz (2006). "The B12 anti-tryptase monoclonal antibody disrupts the tetrameric structure of heparin-stabilized beta-tryptase to form monomers that are inactive at neutral pH and active at acidic pH". J Immunol. 176 (5): 3165–72. PMID 16493076.
- ^ Y. Fukuoka and L. B. Schwartz (2007). "Active monomers of human beta-tryptase have expanded substrate specificities". Int Immunopharmacol. 7 (14): 1900–8. PMID 18039527.
- ^ J. Hallgren, D. Spillmann and G. Pejler (2001). "Structural requirements and mechanism for heparin-induced activation of a recombinant mouse mast cell tryptase, mouse mast cell protease-6: formation of active tryptase monomers in the presence of low molecular weight heparin". J Biol Chem. 276 (46): 42774–81. PMID 11533057.
- ^ N. M. Schechter, E. J. Choi, T. Selwood and D. R. McCaslin (2007). "Characterization of three distinct catalytic forms of human tryptase-beta: their interrelationships and relevance". Biochemistry. 46 (33): 9615–29. PMID 17655281.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ N. M. Schechter, G. Y. Eng, T. Selwood and D. R. McCaslin (1995). "Structural changes associated with the spontaneous inactivation of the serine proteinase human tryptase". Biochemistry. 34 (33): 10628–38. PMID 7654717.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ L. B. Schwartz (1994). "Tryptase: a mast cell serine protease". Methods Enzymol. 244: 88–100. PMID 7845247.
- ^ M. C. Strik, A. Wolbink, D. Wouters, B. A. Bladergroen, A. R. Verlaan, I. S. van Houdt, S. Hijlkema, C. E. Hack and J. A. Kummer (2004). "Intracellular serpin SERPINB6 (PI6) is abundantly expressed by human mast cells and forms complexes with beta-tryptase monomers". Blood. 103 (7): 2710–7. PMID 14670919.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ a b A. Kozik, J. Potempa and J. Travis (1998). "Spontaneous inactivation of human lung tryptase as probed by size-exclusion chromatography and chemical cross-linking: dissociation of active tetrameric enzyme into inactive monomers is the primary event of the entire process". Biochim Biophys Acta. 1385 (1): 139–48. PMID 9630576.
- ^ R. Alzani, E. Cozzi, A. Corti, M. Temponi, D. Trizio, M. Gigli and V. Rizzo (1995). "Mechanism of suramin-induced deoligomerization of tumor necrosis factor .alpha". Biochemistry. 34 (19): 6344–6350. PMID 7756262.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ A. Corti, G. Fassina, F. Marcucci, E. Barbanti and G. Cassani (1992). "Oligomeric tumour necrosis factor alpha slowly converts into inactive forms at bioactive levels". Biochem J. 284 (3): 905–910. PMID 1622406.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ R. Hlodan and R.H. Pain. (1995). "The folding and assembly pathway of tumour necrosis factor TNF alpha, a globular trimeric protein". Eur. J. Biochem. 231 (2): 381–7. PMID 7635149.
- ^ a b c d K. F. Jensen and B. Mygind (1996). "Different oligomeric states are involved in the allosteric behavior of uracil phosphoribosyltransferase from Escherichia coli". Eur J Biochem. 240 (3): 637–45. PMID 8856065. Cite error: The named reference "pmid8856065" was defined multiple times with different content (see the help page).
External Links[edit]
Morpheeins Database [1]
