User:Amrae43/Onchocerciasis
According to the Center for Disease Control and Prevention, as of 2017, about 20.9 million people were infected with river blindness, and an estimated 1.15 million have some amount of loss of vision from the infection. Most infections occur in sub-Saharan Africa, although cases have also been reported in Yemen and isolated areas of Central and South America. In 1915, the physician Rodolfo Robles first linked the worm to eye disease. It is listed by the World Health Organization (WHO) as a neglected tropical disease. In 2013 Colombia became the first country to eradicate the disease.
Causes - Audrey[edit]
Onchocerciasis is a parasitic infection caused by the roundworm species Onchocerca volvulus. The larvae of O. volvulus enter a human host when an infected female adult fly from the family Simulium bites them. After that, it can take up to three months for the worms to mature under the skin of its host.[1] The worms mainly get nutrients for growth in humans from blood, but they have also been seen to rely on other bodily fluids such as cerebrospinal fluid, and urine.[1] It is common to see nodules formed in the skin where the adult worms reside and mate. However, these worms will often travel throughout the body using blood vessels in connective tissues and will even settle behind the cornea.[2]
Epidemiology - Josh[edit]
Cities in Nigeria, Cameroon, Ethiopia, Uganda, and the Congo by far have had the largest amount of infected individuals.[1]
The efforts of CDTI do not go unnoticed as they were able to decrease the number of microfilariae (larvae) loads. This was able to decrease the number of blind people due to onchocerciasis dramatically. However, another issue that arises is the fact that onchocerciasis is able to cause epilepsy, most likely because the level of microfilariae load required to develop epilepsy is much lower than to develop blindness.[3]
Prevention (elimination) - Maddie[edit]
Various control programs aim to stop onchocerciasis from being a public health problem. [4] The Onchocerciasis Control Programme (OCP) launched in 1974, and at its peak, covered 30 million people in the following countries: Benin, Burkina Faso, Côte d'Ivoire, Ghana, Togo, Mali, and Niger. The OCP utilized the following initiatives: the use of larvicide spraying into fast-flowing rivers to control black fly populations, and from 1988 onwards, the use of ivermectin to treat infected people as a core treatment therapy. Alongside the OCP, a joint effort of the World Health Organization, the World Bank, the United Nations Development Programme, and the UN Food and Agriculture Organization, was considered to be a success in controlling onchocerciasis, and in 2002 shifted from control of onchocerciasis to elimination. According to the World Health Organization, four countries have eradicated onchocerciasis that include: Colombia (2013), Ecuador (2014), Mexico (2015), and Guatemala (2016). Continued monitoring ensures onchocerciasis cannot reinvade the area through the OCP.[5] Other effective prevention efforts include personal protection from black fly bites. Recommended protection measures from the CDC include using insect repellents and wearing long sleeves and pants to eliminate exposed skin. Using insect repellent that contains N,N-Diethyl-meta-toluamide (DEET) as well as clothing treated with permethrin.[6]
Elimination
In 1995, the African Programme for Onchocerciasis Control (APOC) initiated to eliminate onchocerciasis from African countries in which the disease was endemic.[7] The initiative relied primarily on the use of the antiparasitic drug ivermectin. The initiative was to set up community-directed treatment with ivermectin for those at risk of infection. Overall transmission has declined.[8] The APOC ended in 2015 and aspects of its work has been taken over by the WHO Expanded Special Programme for the Elimination of Neglected Tropical Diseases (ESPEN). As in the Americas, the objective of ESPEN is working with Government Health Ministries and partner non-governmental organizations, toward elimination of transmission of onchocerciasis. This requires consistent annual treatment of 80% of the population in endemic areas for at least 10–12 years, the life span of the adult worm. No African country has so far verified elimination of onchocerciasis, but treatment has stopped in some areas (e.g. Nigeria), following epidemiological and entomological assessments that indicated that no ongoing transmission could be detected.[9]
In 1992, the Onchocerciasis Elimination Programme for the Americas (OEPA), was launched.[10]On July 29, 2013, the Pan American Health Organization (PAHO) announced that after 16 years of efforts, Colombia had become the first country in the world to eliminate onchocerciasis.[11] Countries that received verification of elimination were Colombia in 2013, Ecuador in 2015, and Guatemala in 2016.[12] The key factor in elimination was mass administration of ivermectin. The OEPA projection was that the disease would be eliminated from all remaining countries in the Americas by 2012.[13] In September 2015, the OEPA announced that onchocerciasis only remained in a remote region on the border of Brazil and Venezuela.[14] The area is home to the Yanomami indigenous people.
No vaccine to prevent onchocerciasis infection in humans is available. This is due to two potential target product profiles (TPPs) that have to be in consideration when developing a vaccine for onchocerciasis.[15] This includes the development of a preventive vaccine for use in children five years or less in age, as this population does not receive ivermectin. Development of a therapeutic vaccine is also necessary that targets adult worms, microfilariae, and the causative agents of pathology and transmission, or both, for children and adults with O. volvulus infection.[16]
Signs and Symptoms - Anni[edit]
It is possible for the larvae to move through the body without triggering a response from the host’s immune system, so some people who are infected with the parasite experience no symptoms; the Global Burden of Disease Study estimated that in 2017 there were at least 20.9 million people infected worldwide, of which 14.6 million had skin disease symptoms and 1.15 million experienced symptoms that impacted vision[17]. After a blackfly bite, it can take 12-18 months for the larvae to develop into mature adult worms that will produce their own larvae, which is what leads to the development of symptoms[18]. Almost all the clinical manifestations of onchocerciasis are due to localized host inflammatory responses to dead or dying microfilariae (larvae)[19]. The signs and symptoms of onchocerciasis are usually divided into two categories, skin and eye symptoms.
Skin symptoms will develop years before any vision problems. These symptoms include[20]:
Intense itchingSwellingInflammationDepigmentationHyperpigmentationRashNodules under the skinSkin atrophyHanging groin (folds of inelastic atrophic skin in the groin associated with enlarged lymph nodes)
Eye symptoms include[21]:
Vision impairment, low vision, or permanent blindness.Clouding of the corneaLight sensitivityLesions on eyesGlaucomaEye painEye redness
Eye symptoms provides the common name associated with onchocerciasis, river blindness, and may involve any part of the eye from conjunctiva and cornea to uvea and posterior segment, including the retina and optic nerve.[22] The microfilariae migrate to the surface of the cornea. Punctate keratitis occurs in the infected area. This clears up as the inflammation subsides. However, if the infection is chronic, sclerosing keratitis can occur, making the affected area become opaque. Over time, the entire cornea may become opaque, thus leading to blindness. Some evidence suggests the effect on the cornea is caused by an immune response to bacteria present in the worms.[23]
Mazzotti reaction[edit]
The Mazzotti reaction, first described in 1948, is a symptom complex seen in patients after undergoing treatment of onchocerciasis with the medication diethylcarbamazine (DEC). Mazzotti reactions can be life-threatening, and are characterized by fever, urticaria, swollen and tender lymph nodes, tachycardia, hypotension, arthralgias, oedema, and abdominal pain that occur within seven days of treatment of microfilariasis[24].
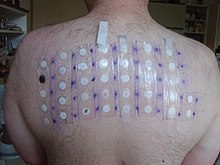
The phenomenon is so common when DEC is used that this drug is the basis of a skin patch test used to confirm that diagnosis. The drug patch is placed on the skin, and if the patient is infected with O. volvulus microfilaria, localized pruritus and urticaria are seen at the application site.[25]
Nodding disease[edit]
This is an unusual form of epidemic epilepsy associated with onchocerciasis although definitive link has not been established.[26] This syndrome was first described in Tanzania by Louise Jilek-Aall, a Norwegian psychiatric doctor in Tanzanian practice, during the 1960s. It occurs most commonly in Uganda and South Sudan. It manifests itself in previously healthy 5–15-year-old children, is often triggered by eating or low temperatures and is accompanied by cognitive impairment. Seizures occur frequently and may be difficult to control. The electroencephalogram is abnormal but cerebrospinal fluid (CSF) and magnetic resonance imaging (MRI) are normal or show non-specific changes. If there are abnormalities on the MRI, they are usually present in the hippocampus.
Diagnosis/Treatment - Hunter[edit]
Diagnosis
When a clinical diagnosis of onchocerciasis is obtained, doctors take small snips of skin containing 3-5 mg of skin tissue.[27] The skin samples taken are only from the upper dermis.[28] These samples will then be soaked in saline and examined underneath a microscope to check for the presence of microfilaria. If the number of microfilaria is undetectable in the samples, the Mazzotti test is then used. In this test, 6 mg of diethylcarbamazine is administered to the affected area. If the patient experiences intense inflammation or itching in the affected area Microfilaria is present.[27]
Treatment
When treating onchocerciasis there is only one effective drug called ivermectin. The drug works by reducing the release of larvae from the adult worm but the drug does not kill it.[27] Patients taking the drug for the treatment of onchocerciasis may have adverse effects within 1-2 days after the drug is administered. Symptoms of urticaria, pruritus, fever, dermatitis, myalgia, urticaria, swelling of face and limbs, or postural hypotension.[28]
Notes:[edit]
The information regarding Nodding Disease seems thrown together, not cohesive.
The section titled "Cause" might be able to be expanded on
Spelling errors in the "diagnosis" section
Check on References and citations throughout the "Prevention" section & there is some repeated information
Many citations needed in "Epidemiology" section
| This is the sandbox page where you will draft your initial Wikipedia contribution.
If you're starting a new article, you can develop it here until it's ready to go live. If you're working on improvements to an existing article, copy only one section at a time of the article to this sandbox to work on, and be sure to use an edit summary linking to the article you copied from. Do not copy over the entire article. You can find additional instructions here. Remember to save your work regularly using the "Publish page" button. (It just means 'save'; it will still be in the sandbox.) You can add bold formatting to your additions to differentiate them from existing content. |
Switch Cause and Signs & Symptoms sections
Article Draft[edit]
Lead[edit]
Article body[edit]
References[edit]
- ^ a b c Burnham, Gilbert (May 1998). "Onchocerciasis". The Lancet. 351 (9112): 1341–1346. doi:10.1016/S0140-6736(97)12450-3.
- ^ Dalton, Mark (2001). "Onchocerca volvulus". Animal Diversity Web. Retrieved 11/18/2023.
{{cite web}}: Check date values in:|access-date=(help)CS1 maint: url-status (link) - ^ Colebunders, Robert; Nelson Siewe, F.J.; Hotterbeekx, An (March 2018). "Onchocerciasis-Associated Epilepsy, an Additional Reason for Strengthening Onchocerciasis Elimination Programs". Trends in Parasitology. 34 (3): 208–216. doi:10.1016/j.pt.2017.11.009.
- ^ "Onchocerciasis (river blindness)". www.who.int. Retrieved 2023-11-16.
- ^ "WHO | Onchocerciasis Control Programme (OCP)". web.archive.org. 2009-11-24. Retrieved 2023-12-10.
- ^ Prevention, CDC-Centers for Disease Control and (2019-09-09). "CDC - Onchocerciasis - Prevention & Control". www.cdc.gov. Retrieved 2023-12-10.
- ^ Amazigo, U. (September 2008). "The African Programme for Onchocerciasis Control (APOC)". Annals of Tropical Medicine and Parasitology. 102: 19–22. doi:10.1179/136485908X337436. ISSN 0003-4983. PMID 18718149.
- ^ "WHO | African Programme for Onchocerciasis Control (APOC)". web.archive.org. 2009-08-28. Retrieved 2023-12-10.
- ^ Rebollo, Maria P; Zoure, Honorat; Ogoussan, Kisito; Sodahlon, Yao; Ottesen, Eric A; Cantey, Paul T (March 2018). "Onchocerciasis: shifting the target from control to elimination requires a new first-step—elimination mapping". International Health. 10 (Suppl 1): i14–i19. doi:10.1093/inthealth/ihx052. ISSN 1876-3413. PMC 5881272. PMID 29471341.
- ^ "WHO | Onchocerciasis Elimination Program for the Americas (OEPA)". web.archive.org. 2011-04-16. Retrieved 2023-12-10.
- ^ "News Scan for Jul 30, 2013 | CIDRAP". www.cidrap.umn.edu. 2013-07-30. Retrieved 2023-12-10.
- ^ "Onchocerciasis (river blindness)". www.who.int. Retrieved 2023-12-10.
- ^ Sauerbrey, M. (July 2013). "The Onchocerciasis Elimination Program for the Americas (OEPA)". Annals of Tropical Medicine & Parasitology. 102 (sup1): 25–29. doi:10.1179/136485908X337454. ISSN 0003-4983.
- ^ Staff (2015-09-29). "Brazil and Venezuela border is the last place in the Americas with river blindness". Outbreak News Today. Retrieved 2023-12-10.
- ^ "Target product profiles". www.who.int. Retrieved 2023-12-10.
- ^ Hotez, Peter J.; Bottazzi, Maria Elena; Zhan, Bin; Makepeace, Benjamin L.; Klei, Thomas R.; Abraham, David; Taylor, David W.; Lustigman, Sara (2015-01-29). "The Onchocerciasis Vaccine for Africa—TOVA—Initiative". PLoS Neglected Tropical Diseases. 9 (1): e0003422. doi:10.1371/journal.pntd.0003422. ISSN 1935-2727. PMC 4310604. PMID 25634641.
{{cite journal}}: CS1 maint: unflagged free DOI (link) - ^ "Onchocerciasis FAQs". Center for Disease Control. CDC. Retrieved 18 November 2023.
- ^ "Onchocerciasis FAQs". Center for Disease Control. Retrieved 18 November 2023.
- ^ Burnham, Gilbert (May 2, 1998). "Onchocerciasis" (PDF). The Lancet. 351: 1341–1346. Retrieved 18 November 2023.
- ^ "Defeating River Blindness - USF Magazine Summer 2013". dev.magazine.usf.edu. Retrieved 2023-12-11.
- ^ "Onchocerciasis (River Blindness)". Cleveland Clinic. Retrieved 18 November 2023.
- ^ Cite error: The named reference
SSMJwas invoked but never defined (see the help page). - ^ Cite error: The named reference
Francesca2011was invoked but never defined (see the help page). - ^ Babalola, Olufemi (2011). "Ocular onchocerciasis: current management and future prospects". Clinical Ophthalmology. 5: 1479–1491. doi:10.2147/OPTH.S8372. PMID 22069350. Retrieved 18 November 2023.
{{cite journal}}: CS1 maint: unflagged free DOI (link) - ^ "Mazzotti Reaction | MicrobLog: Microbiology Training". Archived from the original on 2010-12-20. Retrieved 2011-01-25.
- ^ Dowell SF, Sejvar JJ, Riek L, Vandemaele KA, Lamunu M, Kuesel AC, Schmutzhard E, Matuja W, Bunga S, Foltz J, Nutman TB, Winkler AS, Mbonye AK (2013). "Nodding syndrome". Emerg Infect Dis. 19 (9): 1374–3. doi:10.3201/eid1909.130401. PMC 3810928. PMID 23965548.
- ^ a b c Burnham, Gilbert (May 1998). "Onchocerciasis". The Lancet. 351 (9112): 1341–1346. doi:10.1016/S0140-6736(97)12450-3.
- ^ a b Klarmann-Schulz, Ute (2017-09-12). "Treatment of lymphatic filariasis and onchocerciasis – current and new therapeutic approaches". dx.doi.org. Retrieved 2023-11-21.
