Wikipedia:Reference desk/Archives/Science/2008 September 18
| Science desk | ||
|---|---|---|
| < September 17 | << Aug | September | Oct >> | September 19 > |
| Welcome to the Wikipedia Science Reference Desk Archives |
|---|
| The page you are currently viewing is an archive page. While you can leave answers for any questions shown below, please ask new questions on one of the current reference desk pages. |
September 18[edit]
dous anyone know how a solar panal works without the who made it stuff?[edit]
The science fair is coming up soon and i cant find any info on a way that a solar panal works theres all these words that lead to another word that lead to another word isthere an easy explination ive checked everywhere and it says stuff about photons electrons and stuff and it dousnt say how the electricity is created if anybody can help me on this with an easy explination please answer —Preceding unsigned comment added by Arkamond (talk • contribs) 00:42, 18 September 2008 (UTC)
- There's a quite plain-language "Simple explanation" in the "Theory" section of the Solar cell page. Remember that electricity is just flowing electrons. DMacks (talk) 00:48, 18 September 2008 (UTC)
- You will need to understand what a Photon and an Electron is before you understand how a solar panel works. If you can't understand at least the introductions to those articles then you may prefer the Simple English Wikipedia. (Simple English articles on Photon and Electron ).
- Hope this helps. APL (talk) 01:06, 18 September 2008 (UTC)
- "Photons" are little packets of light. "Electrons" are little packets of electricity. Basically. The photons (light) from the sun hits the special materials. These materials are special because of photons of the right sort (those from the sun, for example) hit them, they will knock loose electrons from the atoms in the material. These loose electrons run through the material—and moving electrons are basically an electric current. This is then siphoned off and fed into an electrical system as generated power. Does that help at all? --98.217.8.46 (talk) 01:12, 18 September 2008 (UTC)
COCONUT SEEDS[edit]
WHAT HAPPENS TO A COCONUT SEEN IN THE WATER?
HOW DOES A COCONUT SEED'S ABILITY TO FLAT HELP A NEW COCONUT TREE TO GROW?
HOW MIGHT THE HARD SHELL OF A COCONUT HELP A NEW COCONUT TREE GROW? —Preceding unsigned comment added by 71.246.121.96 (talk) 00:55, 18 September 2008 (UTC)
- I'm afraid you're going to have to do your own homework. But you might be interested in reading our article on Coconuts. APL (talk) 01:08, 18 September 2008 (UTC)
WHAT? - WE CAN'T HEAR YOU! Oh, that's better ;-) -hydnjo talk 02:25, 18 September 2008 (UTC)
- (Meaning: please don't type in all capitals, it's often considered rude or angry on the internet:) If you have any questions after reading our article on Coconuts, please come back here and ask them. Franamax (talk) 07:38, 18 September 2008 (UTC)
Transparent and reflective at the same time ![edit]
|
This question inspired an article to be created or enhanced: |
Is there any kind of glass that is transparent to light rays when light incident on it from one side and reflect light rays when light is incident upon the glass from the opposite side ? —Preceding unsigned comment added by Shamiul (talk • contribs) 04:56, 18 September 2008 (UTC)
- In theory no, the principle of reciprocity holds. But you can get semireflective mirrors that reflect 80% and let through 20%. When the other side is dark, like sunglasses, you can see out through it. But you can get different effects if the angle of incidence is different, as in total internal reflection. Graeme Bartlett (talk) 05:13, 18 September 2008 (UTC)
- Two-way mirror? Clarityfiend (talk) 07:25, 18 September 2008 (UTC)
- Also called a one-way mirror. Yes, that's what Graeme is talking about. --Anonymous, 09:15 UTC, September 18, 2008.
- It is actually possible to construct a device which transmits (nearly) totally in one direction and reflects (nearly) totally in the other direction, albeit only at a single wavelength. Such a device is called an optical isolator. If anyone is wondering whether these can violate the second law of thermodynamics, the (short) answer is: no. This is elaborated upon in the optical isolator article and the references therein. 58.96.70.254 (talk) 13:49, 18 September 2008 (UTC)
- I don't think that's quite true. You can build an optical isolator that transmits in one direction and absorbs in the other direction, but one that reflects in the other direction would violate the second law for the reasons discussed in the article's first reference (a blackbody surrounded by such isolators would cool to absolute zero). -- BenRG (talk) 15:49, 18 September 2008 (UTC)
- Oops, my bad. The article's first reference is quite explicit about this distinction, yet I still missed it. 58.96.70.254 (talk) 23:34, 18 September 2008 (UTC)
- It actually isn't explained in the article. Someone with more knowledge than me ought to beef up the last paragraph, which skirts the issue quite bluntly. Plasticup T/C 15:44, 18 September 2008 (UTC)
- I added a very brief summary of Rayleigh's argument there. What do you think of it? --Tardis (talk) 16:36, 19 September 2008 (UTC)
- Hah. I've read Rayleigh's argument twice and didn't understand it (and I've taken an intermediate optics course, no less). But now it makes sense; thanks! Someguy1221 (talk) 16:50, 19 September 2008 (UTC)
- I added a very brief summary of Rayleigh's argument there. What do you think of it? --Tardis (talk) 16:36, 19 September 2008 (UTC)
- It actually isn't explained in the article. Someone with more knowledge than me ought to beef up the last paragraph, which skirts the issue quite bluntly. Plasticup T/C 15:44, 18 September 2008 (UTC)
Lithium Batteries (Elephant Tripling)[edit]
I'm not coming to this pure, I just watched Colbert interview Bob Lutz, who kinda trashed his own battery vehicle , but it's a question I've been pondering. It seems that for hybrid and EV passenger cars to progress: lead-acid batteries won't cut it based on the power/weight ratio; NiMH is too limited (dendrification?); and lithium Lilon batteries are the way to go.
The question being, where does the lithium come from and is there enough of it to supply the massive demand for EVs? No question there's enough for cellphones and other small rechargeable batteries, but where is the source for really large demand? Let's say just a million vehicles per year and the amount of lithium in each battery is ?how much? I've found this, which is pessimistic, and I've also seen questions about lithium availability raised reliably. Does anyone have some good sources? Franamax (talk) 07:17, 18 September 2008 (UTC)
- Read everything on [U.S. Geological Survey: Lithium Statistics ]. It should give more than enough info.--Stone (talk) 12:45, 18 September 2008 (UTC)
- World production 2007 25,000 t while worl resources are 13 million t (520 times).--Stone (talk) 12:47, 18 September 2008 (UTC)
- If electric vehicles (or hybrids) are to become the mainstream mode of transport then recycling the batteries will become a major part of the product lifecycle. The lithium in a LiON cell doesn't get "used up" or "worn out" - so all we need is a means to ensure that spent and damaged cells actually DO get recycled. Then all we'll need is a small supply of Lithium to catch those few batteries that somehow don't make it back to the recycling center. Initially, we'll need legislation to ensure that this does actually happen - otherwise we'll end up running out of Lithium first and THEN panicking to find the old ones by mining landfill sites - and that would be horrifically costly!
- World production 2007 25,000 t while worl resources are 13 million t (520 times).--Stone (talk) 12:47, 18 September 2008 (UTC)
- However, I doubt that LiON batteries will remain the best technology for long - there is always another kind of battery in the technology pipeline. SteveBaker (talk) 14:02, 18 September 2008 (UTC)
- Really? Lithium batteries are the first new consumer grade rechargable in 20 years. (To be fair, NiMH batteries only became cheap enough to have widespread consumer use about a decade ago, but their technology was not fundementally new.) The technological evolution of batteries has been far slower than most other forms of technology. If history is a guide, we probably won't have a superior new type of consumer rechargable battery (i.e. one not based on Li) for 10-20 years, and you could see an awful lot of hybrids during 20 years. Probably the disruptive technology with the best chance of replacing Li batteries is not a battery at all, but rather ultracapacitors. However those don't yet store practical amounts of power at reasonable prices (not to mention that pesky blowing-up problem many of them seem to have). Dragons flight (talk) 14:48, 18 September 2008 (UTC)
Edible fir cones / pine cones[edit]
I asked this question on the miscellaneous section but the answers were not satisfactory and I hope you scientists can help. Last week in Istanbul, Turkey, I noticed in food shops, bottles of fir cones for sale. All were closed (immature) but some as long as three inches and perhaps two inches wide. I presume they were in some sort of brine or vinegar. My question is - how can wood impregnated with resin be made edible? (I use fir cones as fuel.) If this is truly the case, a vast food source is going unharvested worldwide. With thanks. —Preceding unsigned comment added by 77.199.89.144 (talk) 08:56, 18 September 2008 (UTC)
- Well pine nuts are certainly edible and are sold in the shops where I live. According to our pine cone article some species of cone are edible including some species of Podocarpaceae. SpinningSpark 09:14, 18 September 2008 (UTC)
Thank you for the replies. I looked at the pine cone article but, unfortunately, the sort of 'cones' that appear to be edible are soft and berry-like, like yew, not remotely like the conventional hard pine cones I saw. The second reply is intriguing. So, the water is not drunk but used - what for please? —Preceding unsigned comment added by 86.67.202.50 (talk) 14:33, 22 September 2008 (UTC)
- Yew is very poisonous - do not eat! 78.147.10.10 (talk) 21:36, 24 September 2008 (UTC)
Conversion[edit]
What is the right conversion factor to be use in obtaining the volume of liquid CO2 from a pressure guage (pressure guage indicate the H2O content of the horizontal cylindrical tank). Note: Dimension of the tank is known. —Preceding unsigned comment added by Sheann (talk • contribs) 10:22, 18 September 2008 (UTC)
- My guess is that the pressure gauge is reading in cmH20 (not water content). I don't think that the pressure of the gas will vary in a direct way with the volume of liquid below. Perhaps there's some detail I am missing. --Scray (talk) 11:04, 18 September 2008 (UTC)
- The article on Torr#Manometric_units_of_pressure may help you with conversion factors. --Scray (talk) 11:12, 18 September 2008 (UTC)
- The articles on vapor and vapor pressure may also be useful. --Scray (talk) 11:36, 18 September 2008 (UTC)
- I would think it impossible to determine the volume of liquid from a pressure gauge at the top of the tank. If you can measure the pressure of the liquid at the bottom too - then you could use the difference between the pressure at the top of the tank and the pressure at the bottom to determine the depth of the liquid - and from that you can know the volume if you know the shape of the tank.
- Personally, I'd just weigh the tank empty and full and go from there. SteveBaker (talk) 13:55, 18 September 2008 (UTC)
- That's a good approximation, since most of the mass is probably in liquid form, but the OP should be aware that they are actually measuring the mass of the gas too. The pressure can not directly indicate the volume of anything - but it can indicate the mass-fraction (ratio of liquid to gas), if the temperature is known. Nimur (talk) 15:14, 18 September 2008 (UTC)
- Personally, I'd just weigh the tank empty and full and go from there. SteveBaker (talk) 13:55, 18 September 2008 (UTC)
Labelling and imaging with non-radioactive isotopes?[edit]
I'm a student soon to go to work at a research institute where I'll be looking at the cellular of activity of small molecules which are made in-house. I would like to gain some insight by labelling these small molecules. Fluorescent labels would be as big as, or larger than the molecules of interest so that's out of the question. I'm left with radioactive labels or the use of non-radioactive isotopes, such as deuterium or carbon-13 etc. I was wondering if someone could advise on the relative cost, and practicality of using a radioactive versus non-radioactive isotopes. Since I would want to be able to visualise the molecules in a gel after electrophoresis, it may not even be possible to use non-radioactive isotopes (I don't know how you'd detect the presence/location of deuterium in a gel). I'd like to develop my ideas a little before they're shot down by my supervisor. :) ----Seans Potato Business 13:30, 18 September 2008 (UTC)
- Even non-radioactive isotopes will change the mass profile on a mass spectrometer, which is suitable for tagging chemicals. They are also cheaper and easier and safer. If you will be working hands-on with chemicals you're unfamiliar with, you should probably directly ask your supervisor for training, as safety and exposure hazards might exist. Nimur (talk) 15:11, 18 September 2008 (UTC)
- I want to take whole cell lysates and separate them by 2D PAGE, then image them. Since we've no clue where the molecules are binding, I don't think mass spectrometry is a feasible means to find out. I want to use mass spec. to identify the relevant spots from the gel, but first they must be imaged. Non-radioactive isotopes ought to be perfectly safe, even if somewhat expensive, right? Their safety relative to radioactive ones, is why I'm interested in whether I can image them. ----Seans Potato Business 22:11, 18 September 2008 (UTC)
- The safety of rare non-radioactive isotopes is exactly equivalent to that of the natural, high-abundance isotopes. Carbon-13 is no more dangerous than carbon-12. Incidentally, are you expecting your molecules of interest to be covalently linked to their protein partners? While there are some non-covalent interactions that may survive 2D-PAGE (biotin-(strept)avidin, for example) I wouldn't rely on this happening. Short of staining your entire 2D gel for protein (silver stain, Coomassie, Sypro orange, etc.) and cutting out every spot for analysis, I can't think of any reliable way to find a non-radioactive target.
- I don't suppose an antibody exists (or could be prepared, depending on your timetable and budget) to your small molecule of interest...? If you can buy one from the catalog, you're laughing.
- I'm a bit unsure about exactly what type of experiment you're doing, though. Are you looking for something that gets covalently bound to protein? Something that's transported? A drug that fills a binding pocket? Something that gets processed or modified? Are you using something that's present in nature? An analog? Something from a different organism? Something totally weird and synthetic? Do you expect to find a lot or a little interaction? Do you have microliters, milliliters, or liters of lysate? The best strategies are going to depend a lot on what you're studying and what you're hoping to find out. If you're synthesizing stuff in-house anyway, does your molecule have a non-functional face to which you can attach a flexible linker (PEG, maybe) and a fluorophore? (Do you have a collaborator who likes organic synthesis?) TenOfAllTrades(talk) 23:09, 18 September 2008 (UTC)
- If as you said even a small fluorescent tag is going to be a problem (not even a quinone or anthracene?!), I don't think you have too much wiggle-room for anything except isotopic labeling of some sort. The alternatives are "some sort of chemically reactive site" that could be selectively stained directly or via some affinity probe, but it would have to be pretty active to be detected on a small scale (and hence likely to be reactive in your cell goo). You could embed some heavy-metal and then look for redox activity , or use some sort of particle-beam scanner (a la ECD or neutron bombardment) but then you're back to radioactivity.
- Organic chemistry could be fun. Do you have even enough space to attach an azide? Do come Sharpless click chemistry to attach a fluorophore after lysis. Or heck, even after running the gel if you can get it to work in a gel medium (and therefore not have the fluorophore alter your mobility). DMacks (talk) 23:35, 18 September 2008 (UTC)
- 2D-PAGE has a reputation for being finicky and poorly reproducible. If you're going the mass spec route, there's been some interesting work done recently with two-dimensional HPLC/Electrospray ionization/tandem mass spectrometry, especially the studies with Isotope Coded Affinity Tags (ICAT), Stable Isotope Labeling Tandem mass spectrometry (SILT), and Stable Isotope Labeling with amino acids in Cell culture (SILAC). Protein mass spectrometry indicates that 2D-PAGE is also used as a separation technique, usually with MALDI mass spec. I will note that as long as you already have the appropriate regulatory clearances and equipment, radioisotopes are perfectly safe and, depending on the isotope, as cheap as stable labels. However, if you have to synthesize the chemicals in-house, the organic chemists doing the synthesis might get a little worried at working with radioactivity. (If they aren't used to doing such things, I probably wouldn't consider asking them to). However, I'm a little confused at your statements - are you expecting these molecules to form covalent bonds to the protein? Because if not, the HPLC/PAGE separations are likely to rip the ligand from it's partner long before you get a chance to image it. If you're looking for non-covalent binding, you're probably going to have to attach a cross-linking group on it before chromatography, either a side-chain reactive group like iodoacetamide or N-Hydroxysuccinimide, or a photoreactive crosslinker like benzophenone or an aryl azide. Another alternative to identify binding partners is to link your small molecule to a solid support, and do affinity chromatography fishing through lysates. I've also seen a Three-hybrid type approach where a small molecule conjugate replaces the RNA. The last two techniques aren't in native contexts, though. -- 128.104.112.147 (talk) 00:12, 19 September 2008 (UTC)
Newton phoney quote?[edit]
There's what looks like a phoney quote of Newton going around on creationist websites that's been said to me : one version goes -
- Newton once had a skilled mechanic make for him a model of the solar system. Balls representing the planets were geared together so as to move realistically in orbit. One day an atheist friend visited Newton. On seeing the model, he operated it, and exclaimed in admiration, "Who made it?" Newton answered, "Nobody!" The atheist replied, "You must think I am a fool! Of course somebody made it, and he is a genius." Newton then said to his friend, "This thing is but a puny imitation of a much grander system whose laws you know, and I am not able to convince you that this mere toy is without a designer and maker; yet you profess to believe that the great original from which the design is taken has come into being without either designer or maker!"
Whilst this is by no way an exhaustive search this quote isn't on the wikiquote page for Isaac Newton (http://en.wikiquote.org/wiki/Isaac_Newton). If you've copied it word-for-word from somewhere I would say it is written really quite horribly. It feels (and i'm by no means an expert) like someone writing today trying to add an edge of age by twisting around the flow/sense. It doesn't seem to be real to me. I can't find an article on snopes though which would be my first resource usually. 194.221.133.226 (talk) 15:14, 18 September 2008 (UTC)
- Speaking of Snopes, while this Einstein story isn't exactly the same, I'd like to quote a relevant paragraph:
- Although 2004 tellings of the legend name Albert Einstein as the faith-driven student, there is no reason to suppose the renowned physicist had anything to do with the fictive incident. Biographies of the man are silent on his having dealt one of his teachers such a comeuppance. Moreover, this famous scientist gets used in legends whose plots call for a smart person, one whom the audience will immediately recognize as such (i.e., modern tellings of an ancient legend about a learned rabbi who switches places with his servant feature Albert Einstein in the role of esteemed scholar). This venerated cultural icon has, at least in the world of contemporary lore, become a stock character to be tossed into the fray wherever the script calls for a genius.
- Newton fulfils a similar role here. AlmostReadytoFly (talk) 15:32, 18 September 2008 (UTC)
- Not that it sheds much light on this quote, but our article on Isaac Newton is fantastic. Plasticup T/C 15:40, 18 September 2008 (UTC)
- The Newton story above may be apochryphal, but it is at least consistent with some of his sourced remarks quoted in our article on Isaac Newton's religious views. On the other hand, Newton also had very unorthodox religious opinions for his time; he denied the existence of the Trinity, and spend a lot of time and effort in a quest for the Philosopher's stone and other alchemical pursuits. Even the greatest genius can have some odd hobby horses. Fortunately, the scientific method is based on gathering observable, empirical and measurable evidence, not on appeals to authority ! Gandalf61 (talk) 16:15, 18 September 2008 (UTC)
- The original quote is obviously poorly-rendered Creationist claptrap. Ironically, the fact that an undeniable genius like Newton really was a devout religionist occasionally gives me pause about my own opinions, so any verifiable religious quote from him would actually have been more effective (to a heathen like me) than this more on-point one they invented. --Sean 16:21, 18 September 2008 (UTC)
- On that topic, see also God of the gaps. TenOfAllTrades(talk) 22:35, 18 September 2008 (UTC)
One of the more amusing things about Newton that people have a hard time dealing with today is that when he postulated the idea of a force called "Gravity", a lot of his contemporaries thought it was basically magic he was arguing for, not science. They accused him of appealing to "occult" forces, to things that could not be explained. After all, Newton didn't know why it worked or how it worked, but postulating this invisible, weak force seemed to get the job done in the equations. After some time though the idea of gravity became pretty internalized and nobody doubted it as a real force, and then it waited until Einstein to say that there wasn't a force called gravity after all, that it was just warping of spacetime by mass, etc. Anyway, I always thought that was amusing—the idea that other scientists and philosophers accused Newton of being unscientific. --98.217.8.46 (talk) 13:51, 19 September 2008 (UTC)
- When you say "other scientists and philosophers", it is probably worth noting that in his time scientists were not differentiated from philosophers. What today we call scientists were called "natural philosophers", but their work still fell under the purview of philosophy. Theology, by the same token, was also a branch of philosophy, so science and religion were not each others antitheses as they are to many today. We have an article on the relationship between religion and science, but I haven't the time to read it tonight. Plasticup T/C 04:25, 20 September 2008 (UTC)
Why is lightning blue?[edit]
Pretty simple question, couldn't think of a simple answer..--Shniken1 (talk) 16:00, 18 September 2008 (UTC)
- Is it? I've always seen it as being yellowish, and this page agrees with that. --Sean 16:08, 18 September 2008 (UTC)
- It's always looked white to me. :) --Kurt Shaped Box (talk) 16:14, 18 September 2008 (UTC)
- Not a lighting expert, but the visible part of lightning is the plasma which is formed from superheating air formed by the electric discharge. Like stars, (see Star color#Harvard spectral classification), the color of the blackbody radiation given off is dependent on the temperature of the plasma. The lightning article indicates that temperatures can reach up to 30,000 °C - this is a bluish-white color. Lesser lightning strikes are likely to be cooler, and may give pure white or yellowish-white colorations, depending on the temperature. -- 128.104.112.147 (talk) 18:48, 18 September 2008 (UTC)
- OK, but don't forget that you usually see lightning through many miles of air, which scatters blue light more than warmer colors, which is why it ends up looking yellowish. --Sean 16:09, 19 September 2008 (UTC)
I'm hijacking this question. I saw a Positive lightning bolt once and is there a reason that that lightning bolt was red? —Preceding unsigned comment added by Coolotter88 (talk • contribs) 22:19, 18 September 2008 (UTC)
- See Toto's answer above. If the bolt was so bright you saw it from an unusually large distance, the amount of scattering would be increased. (Just as the rising or setting sun is red because you're seeing it through an unusally large amount of the atmosphere.) --Anonymous, 03:47 UTC, September 23, 2008.
Chromosome/Chromatid[edit]
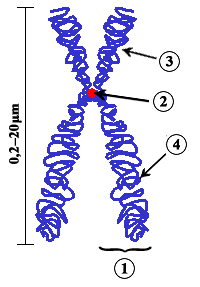
In this image the whole thing is called a chromosome and each strand is known as a chromatid. So when the 2 strands are pulled apart, what are they called? Chromosomes as well? --RMFan1 (talk) 18:31, 18 September 2008 (UTC)
- That question had me stumped for ages as well. The answer is quite simple. Look at the description of the figure " Diagram of a duplicated and condensed (metaphase) eukaryotic chromosome.". The emphasis here is on " duplicated ". Each half is a chromosome. In this picture you see two identical chromosomes.PvT (talk) 18:54, 18 September 2008 (UTC)
- My biology professor hammered this into my brain: when the chromosome is in its familiar x-shape, there is one chromosome, and two identical chromatids. This is what the chromosome looks like during early mitosis. Normally, the chromosomes are single-stranded, so there is one chromosome and one chromatid.CalamusFortis 21:09, 18 September 2008 (UTC)
- I think we've got bad wording in the sister chromatids stub -- the stub seems to agree with PvT's take, but I think Fortis's take is the correct one: the whole x-shaped thing is a chromosome. And as for the OP's question, here's a quote from chromosome: "Chromosomes may exist as either duplicated or unduplicated—unduplicated chromosomes are single linear strands, while duplicated chromosomes (copied during synthesis phase) contain two copies joined by a centromere." So after separation, what was a chromatid is now called a chromosome. --Allen (talk) 06:47, 19 September 2008 (UTC)
So basically, it's what I said:
- X shape thing is a chromosome
- Single strand is also a chromosome
- Individual strands in the X shape thing are chromatids
Right? —Preceding unsigned comment added by RMFan1 (talk • contribs) 10:53, 19 September 2008 (UTC)
erection problems[edit]
Is a man with big penis more likely to have such problems? Has anyone researched into this? —Preceding unsigned comment added by Tony May (talk • contribs) 18:35, 18 September 2008
- Actually it's the opposite. Read this paper. Axl ¤ [Talk] 13:37, 19 September 2008 (UTC)
examples of imperfect human evolution[edit]
Hello,
I read something years ago about examples of imperfect evolution in humans. I.e to prove the point the humans aren't pinnacles of creation- there are things about our physiology which suck. Anyway, i now can't for the life of me remember any examples. Can anyone help?82.22.4.63 (talk) 18:40, 18 September 2008 (UTC)
- Lower backs, knees, teeth that decay, and unnecessary blind spots in the eyes. How long a list do you need? --Stephan Schulz (talk) 19:02, 18 September 2008 (UTC)
They are not actually specific to humans. I would hazard a guess and say all animals with eyes have blindspots, all with teeth have tooth decay, all with knees have, well, knees. And the 'lower back' thing, I am not sure what you are talking about. --ChokinBako (talk) 19:53, 18 September 2008 (UTC)
- No, cephalopods have much more reasonably constructed eyes. I'm fairly sure I read about some animals with decay-proof teeth, or, as an alternative, we could have teeth that auto-replace themselves, as in sharks. Human knees and (especially) the lower back suffer from the fact that they originally evolved for tetrapods which kept their weight distributed and their spine horizontally, not for upright walking. --Stephan Schulz (talk) 20:16, 18 September 2008 (UTC)
- I was going to explain my deletions on Talk page, but find this Desk has no Talk page. I deleted based on the policy WP:BLP. Wanderer57 (talk) 20:11, 18 September 2008 (UTC)
- FYI, the various desks share a talk page at Wikipedia talk:Reference desk. Discussions of deleted content, etc., are entirely appropriate there. -- Coneslayer (talk) 18:43, 19 September 2008 (UTC)
- I was going to explain my deletions on Talk page, but find this Desk has no Talk page. I deleted based on the policy WP:BLP. Wanderer57 (talk) 20:11, 18 September 2008 (UTC)
- The eyeball is a particularly good one, since creationists so often use it as an example of why humans couldn't have evolved (see Irreducible complexity):
- The nerves and blood vessels supplying the retina are in front of the retina, leading to a loss of visual acuity and a blind spot where they pass through the retina.
- The lens of the eye has no mechanism for self-repair, so over the years you get a loss of focus ability, and may develop cateracts
- The light sensors in the eye can't deal with constant-intensity illumination, so your eyes need to continuously wobble to be able to see things.
- The color sensors don't work in low-light conditions, while the monochrome sensors don't work in bright conditions.
- As a consequence of the above, people have trouble in high-contrast situations (say, looking into a cave to see if there's a bear in it).
- If I'd designed the eye, I sure woudn't want my name associated with it. --Carnildo (talk) 21:05, 18 September 2008 (UTC)
The urethra passes through the prostate.[1] --Kjoonlee 00:37, 19 September 2008 (UTC)
I'm sure if you ask any of your female friends whether their reproductive system seems "perfectly designed" or "just the bare minimum needed for a species to survive" I'm sure you'd find most of them believe the latter. I say this with tongue firmly in cheek, but it is not too hard to imagine a female reproductive system that doesn't involve monthly periods (and their waste of energy and material) or a birth process that breaks the pelvis. (My wife and I have discussed this very issue—she loathes her reproductive system.) --98.217.8.46 (talk) 01:37, 19 September 2008 (UTC)
But surely most of these things were positives when we were cavemen? i take your point about the lower back but with the reproductive system, surely all (female) mammals have periods? i.e. they are, whilst no doubt inconvenient for todays career woman, an efficient system to ensure the possibility of lots of babies. I actually can't imagine a better method (although this has more to do with my non-existent understanding of female anything physiology so some info would be appreciated).
Equally the eye faults (apart from that awful pun!) are surely a by-product of the fact that our environments are relatively 'boring'. I.e. we don't need whizz-bang eyes because we usually operate during the day. presumaly there are various trade offs with things like energy requirements but i don't know..(Although it is weird that they have no healing capacity..)217.169.40.194 (talk) 08:45, 19 September 2008 (UTC)
- How about suicidal depression? --68.166.144.211 (talk) 08:56, 19 September 2008 (UTC)
- A blind spot is bad engineering; bad engineering may be be benign, but it's never a positive, and some female mammals absorb their uterine lining. --Kjoonlee 11:51, 19 September 2008 (UTC)
- The human reproductive system is another one of those nightmares of design.
- Almost all sexually-reproducing organisms have either a dedicated breeding season or a signal that a female is fertile. Humans don't, so a great deal of time and energy is wasted copulating with non-fertile females.
- Babies are almost too large for the female reproductive system -- because of this, humans have a much higher rate of maternal mortality than any other species.
- On the same lines, running the birth canal through the pelvis is a horrible design: it limits the maximum size of the newborn, and causes severe problems if things aren't lined up exactly right.
- Placing the reproductive and excretory systems in close proximity can lead to severe infections.
- Again, I wouldn't sign off on such a flawed design. --Carnildo (talk) 20:06, 19 September 2008 (UTC)
- A nitpick: human maternal mortality is high, but not the highest; spotted hyenas have 16% of first-time mothers dying in childbirth (and about 80% of firstborn cubs), whereas even in the most unhygienic regions, human maternal mortality tops out at 1-2% (and that's with modern diseases, etc. - I'm sure it was lower in the Paleolithic, as many of our diseases originated from livestock.) I'm not sure how else you could possibly give birth to big-brained kids, short of doing it in the embryo stage. I'm not sure this is an "evolutionary inertia" issue like the bipedalism-related pains. Vultur (talk) 21:55, 19 September 2008 (UTC)
My back chronically hurts, my eyesight is poor, I get sunburns to even mild exposure to the sun, I have to spend a third of my life sleeping, I'll spare you other details, but whoever created us did a very losy job :P Not even a technical support line to complain :P Equendil Talk 09:03, 19 September 2008 (UTC)
- our mechanism for assessing and avoiding risk is useless for anything short of a direct attack by a tiger. that's a serious lack; consider how many people are killed each year in accidents due entirely to their own stupidity, and how many more are killed from smoking, overeating/lack of exercise, etc. we even have Darwin awards to celebrate it. compare with the Pierson's Puppeteers for an example of actual intelligent design, albeit by a mere science fiction writer. Gzuckier (talk) 15:05, 19 September 2008 (UTC)
- Humans also are rather poor at comprehending large numbers (think about people's difficulty with statistics and or the massiveness of the universe). Granted we evolved for the habitat scale we grew up in. We are also easy influenced, gullible, and trusting/suspicious of the wrong things. Psychological manipulation does have some basis in evolution and not just social conditioning; our minds definitely leave something to be desired. We haven't evolved well to deal with the treat posed by charlatans.
My own personal peeve, the inability to get sharp visual focus at high speeds, even at 30mph, at anything other than straight ahead. —Preceding unsigned comment added by 209.189.246.212 (talk) 22:46, 20 September 2008 (UTC)
- I'm surprised that no one has mentioned the vermiform appendix yet. If the appendix isn't useless, it's damn near. TenOfAllTrades(talk) 22:29, 19 September 2008 (UTC)
Why did Gregor Mendel ("The Gregster") choose the pea to study genetic inheretence?[edit]
Out of all the plants and animals....???.... Seems a little silly doesn't it? Was this a random choice? Or perhaps it occured to him after tipping a few?--Sunburned Baby (talk) 20:22, 18 September 2008 (UTC)
- They're readily available, they've got a decently short lifecycle, they've got some obvious trait variations, and it's easy to control breeding. --Carnildo (talk) 21:07, 18 September 2008 (UTC)
- I thought he just grew peas for pleasure Coolotter88 (talk) 22:16, 18 September 2008 (UTC)
- Remember that he was a monk, so studying a food crop that grew in the abbey garden made sense. (You could eat the experiments, and allowed for the sort of pottering* that a field-grown crop like wheat wouldn't have. It's also easier to grow 4,000+ pea plants a year rather than 4,000+ chickens.) Augustinians (Mendel's order) practice moderation and self denial, so he wasn't likely to have studied some exotic plant just for novelty's sake. Also keep in mind that he didn't set out to study genetics as such (since the discipline didn't exist before Mendel). The Mendel article indicates that his purpose initially was to study variation in plants. This is speculation on my part, but he may have chosen to study peas because he had access to several pea varieties which were noticeably different. Besides, what's so silly about peas? * (No article on pottering? That's curious. See [2] instead.) -- 128.104.112.147 (talk) 23:09, 18 September 2008 (UTC)
Mendel was performing hybridization experiments, which were traditionally used to investigate the problem of the origin of species rather than laws of heredity. Plant hybridization studies were favored by horticulturalists who wanted to learn how to create and fix varieties, and academic studies of hybrids focused on whether new species could be created by cross-breeding existing ones. It has been argued that Mendel was actually trying to oppose evolutionism and instead defend Linnaeus' theory of the production of new species by hybridization. In this, peas were an example of “variable” hybrids, where as Hieracium was an example of "constant hybrids" that were potentially new species.
But yeah. Enough of my notes from ages ago. Why peas? Don't underestimate Mendel as a horticulturalist. He experimented with a number of different plants. Peas gave the best results. (The results were a little too good, in fact—there is evidence that Mendel doctored his figures a bit. But alas.) He was not just some clueless monk who happened to by chance pick a useful species; he considered himself a pretty able scientist. The idea that he was totally out of the loop and happened upon this great insight by chance and was just painfully ignored though ahead of his time is a myth. The actual history is a lot more complicated. Mendel didn't think he was discovering wonderful laws of heredity. A wonderful, short book on the topic is Peter J. Bowler, The Mendelian Revolution: The Emergency of Hereditarian Concepts in Modern Science and Society (1989). --98.217.8.46 (talk) 01:17, 19 September 2008 (UTC)
- Another reason that he used the garden pea is that he was using (in part) the characteristics of the seeds (wrinkled, smooth, etc.) as markers--not of the plant. This meant that he had data as soon as the seeds were formed, without having to plant the seeds to see what the plants would be like (which is what he would have had to do if, for example, he had been using the color of the flower). Here is a relevant quote from an English translation of his 1865 paper [3]: "The hybrid forms of the seed-shape and of the [color of the] albumen are developed immediately after the artificial fertilization by the mere influence of the foreign pollen. They can, therefore, be observed even in the first year of experiment, whilst all the other characters naturally only appear in the following year in such plants as have been raised from the crossed seed." This would not necessarily have been possible with other garden plants, and would probably have been impossible with most domestic animals.--Eriastrum (talk) 17:45, 20 September 2008 (UTC)
genetics[edit]
Are siblings of the same parents closer genetically than father and son? —Preceding unsigned comment added by Babycygnet (talk • contribs) 22:47, 18 September 2008 (UTC)
- It's a draw, at least statistically speaking. If we ignore the X/Y size imbalance, exactly one half of a son's DNA comes from his father, so the father/son pair share ~50% of their genome. In the sibling case, each randomly inherits 50% of the mother's genome, and 50% of the father's. Since this is random, if we consider the half of the DNA I get from my mother, I could inherit the same half of my mother's genome that my sister did (so we share all of the maternal half), or I could inherit the exact opposite half (so we share none), or anywhere in between. Same goes for the father's side. On average, siblings will share 50% from each parent, meaning that they share 50% of the genome overall, although it can range anywhere from 0-100%. Although one issue that slightly tips it towards siblings is the existence of identical twins, who get their identical genomes not from the random assortment, but from a genome duplication. -- 128.104.112.147 (talk) 23:25, 18 September 2008 (UTC)
- Actually, I think you've got your statistics wrong. The OP asked about genetic similarity. The likelihood that a particular gene being present in both siblings is 25% (P=0.5 * 0.5), I believe. -- JSBillings 19:06, 19 September 2008 (UTC)
- No he is perfectly correct. For any particular gene that is present in one sibling the chance of it being in the other sibling is 0.5. Your 0.25 is the chance that both siblings share a particular gene that one or other of the parents have - but of course it is possible neither sibling has it. Dmcq (talk) 19:41, 19 September 2008 (UTC)
- Actually, I think you've got your statistics wrong. The OP asked about genetic similarity. The likelihood that a particular gene being present in both siblings is 25% (P=0.5 * 0.5), I believe. -- JSBillings 19:06, 19 September 2008 (UTC)
What caused these global cooling events?[edit]
The period of transition between the Ordovician and the Silurian, the Carboniferous and the Permian, the Jurassic and the Cretaceous, and the Tertiary and the Quaternary periods appears to be marked by an average global temperature of not more than 12°C. What might have caused this reduction in average global temperature from the average global temperature of 22°C for all other period transitions? 71.100.15.15 (talk) 23:04, 18 September 2008 (UTC)
- Ordovician–Silurian extinction event has some information. Also Permian–Triassic extinction event, Triassic–Jurassic extinction event, etc. Plasticup T/C 06:00, 19 September 2008 (UTC)

