User:Thilini ukwaththage/sandbox
 | This is a user sandbox of Thilini ukwaththage. You can use it for testing or practicing edits. This is not the sandbox where you should draft your assigned article for a dashboard.wikiedu.org course. To find the right sandbox for your assignment, visit your Dashboard course page and follow the Sandbox Draft link for your assigned article in the My Articles section. |
Introduction[edit]
Electron Ionization (EI, formerly known as electron impact) is one of the firstborn molecular ionization technique, used in mass spectrometry. However, this method is still considered as the most popular ionization technique. This technique is considered as a hard ionization method, since it uses high energetic electrons interacting with gas phase atoms or molecules to produce ions. This leads to extensive fragmentation, which can be helpful for structure determination of unknown compounds. EI is the most useful for organic compounds which have a lower molecular weight than 600 Da. Also, several other thermally stable, volatile compounds in solid, liquid and gas state can be detected with the use of this technique coupled with various separation methods.[1]
History[edit]

Electron Ionization was first used in 1918 by Canadian- American Physicist Sir Arthur j. Dempster in the article of “A new method of positive ray analysis”. It was the first modern mass spectrometer, which was used to analysis of positive rays, based on the determination of the ratio of the charge to mass of various constituents.[2] ]. In this method, the ion source was described to be similar to the modern EI method. The anode was made cylindrical in shape using the metal which has to be studied. Subsequently it was heated by a concentric coil and then was bombarded with electrons. Using this method, the two isotopes of lithium and three isotopes of magnesium with their atomic weights and relative proportions were able to be determined.[3]Since then this technique is uses up to now with further modifications and developments.
Principle of operation[edit]

In this process, an electron from the analyte molecule (M) is expelled during the collision process to convert the molecule to a positive ion with an odd number of electrons. The following gas phase reaction describes the electron ionization process[4]
where M is the analyte molecule being ionized, e− is the electron and M+• is the resulting ion called as molecular ion.
In an EI ion source, electrons are produced through thermionic emission by heating a wire filament that has electric current running through it. The kinetic energy of the bombarding electrons should have higher energy than the ionization energy of the sample molecule. Due to this reason, the electrons are accelerated to 70 eV in the region between the filament and the entrance to the ion source block. The sample under investigation which contains the neutral molecules is introduced to the ion source in a perpendicular direction to the electron beam. Close passage of highly energetic electrons in low pressure(ca. 10−5 to 10−6 torr), referred to as a hard ionization source, causes large fluctuations in the electric field around the neutral molecules and induces ionization and fragmentation.[5] The fragmentation in electron ionization can be described using Born Oppenheimer potential curves as in the diagram. The red arrow shows the energy of electron impact energy which is enough to remove an electron from the analyte which can form a molecular ion. Due to the higher energy supplied by 70 eV electrons other than the molecular ion, several other bond dissociation reactions can be seen. It is shown by the blue arrow in the diagram. These ions are called as second-generation product ions. The radical cation products are then directed towards the mass analyzer by a repeller electrode. The ionization process often follows predictable cleavage reactions that give rise to fragment ions which, following detection and signal processing, convey structural information about the analyte.
The efficiency of EI[edit]
To increase the electron ionization process, the easiest way to increase the ionization efficiency. To achieve higher ionization efficiency should be optimized filament current, emission current, and ionizing current. The current supplied to the filament to heat it to incandescent is called as filament current. The emission current, is the current measured between the filament and the electron entry slit. The ionizing current is the rate of electron arrival at the trap. It is a direct measure of the number of electrons in the chamber that are available for ionization.
The sample ion current (I+) is the measurement of the ionization rate. This can be enhanced by manipulation of the ion extraction efficiency (β), the total ionizing
cross section (Qi) , the effective ionizing path length( L), the concentration of the sample molecules([N]) and the ionizing current (Ie). The equation can be shown as follows:
The Ion extraction efficiency (β) can be optimized by increasing the voltage of both repeller and accelaration. Since the ionization cross section depends on the chemical nature of the sample and the energy of ionizing electrons, as a standard value 70eV is used. At low energies (around 20 eV), the interactions between the electrons and the analyte molecules do not transfer enough energy to cause ionization. At around 70 eV, the de Broglie wavelength of the electrons matches the length of typical bonds in organic molecules (about 0.14 nm) and energy transfer to organic analyte molecules is maximized, leading to the strongest possible ionization and fragmentation. Under these conditions, about 1 in 1000 analyte molecules in the source are ionized. At higher energies, the de Broglie wavelength of the electrons becomes smaller than the bond lengths in typical analytes; the molecules then become "transparent" to the electrons and ionization efficiency decreases.The effective ionizing path length (L) can be increased by using a weak magnetic field. But the most practical way to increase the sample current is to operate the ion source at higher ionizing current (Ie).[1]
Instrumentation[edit]
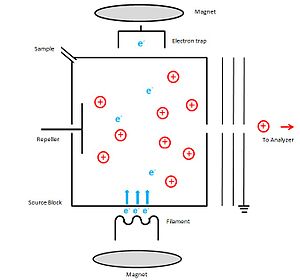
A schematic diagram of instrumentation which can be used for electron Ionization is shown below. Ion source block is made out of metal. As the electron source, the cathode which can be a thin filament of tungsten or rhenium wire has been inserted through a slit to the source block. Then it is heated up to an incandescent temperature to emit electrons. A potential of 70 eV is applied between the cathode and source block, which accelerates the emitted electrons to achieve the kinetic energy which is necessary to make positive ions. The potential of anode (electron trap) is slightly positive and it is placed on the outside of the ionization chamber, directly opposite to the cathode. The unused electrons are collected by this electron trap. Sample is introduced through the sample hole. To increase the ionization process, a weak magnetic field is applied parallel to the direction of the electrons travel. Because of this, electrons travel in a narrow helical path way which increases the path length to perform a collision with an electron. The positive ions that are generated are then repelled by the repeller electrode, to move the ions to the accelerating region through the slit which in the source block. By applying potential to the ion source and maintaining the exit slit at ground potential, ions gain a certain fixed kinetic energy and finally are lead to the mass analyzer. To avoid the condensation of the sample, source block is heated up about 300◦C.[1]
Applications[edit]
Since the early 19th century, electron ionization has been one of most popular ionization technique due to it's tremendous applications. These applications can be broadly categorized by the sample insertion method. The gaseous and highly volatile liquid samples uses the method of vacuum manifold, solids and less volatile liquids use a direct insertion probe and complex mixtures use gas chromatography or liquid chromatography.
Vacuum Manifold[edit]
In this method, the sample is first expanded in to a heated sample reservoir of the vacuum manifold. Then it is escaped into the ionization chamber using a pinhole.
Direct Insertion EI -MS[edit]
In this method, the probe is manufactured from a long metal channel which ends in a well for holding a sample capillary. This is inserted into the source block through a vacuum lock. The sample is introduced to the well using a glass capillary. Then the probe is suddenly heated up to a desired temperature to vaporize the sample. Using this probe, sample can be positioned very close to the ionization region.[1]
Analysis of archaeologic materials[edit]
Direct insertion electron ionization mass spectrometry (Direct insertion EI - MS) was used for the identification of archaeological adhesives such as tars, resins and waxes which can be found during excavations on archaeological sites. These samples are typically investigated using gas chromatography – MS with extraction, purification, and derivatization of the samples. Due to the fact that these samples were deposited in prehistoric periods, they are often preserved in small amounts. By using direct insertion EI – MS archaeological samples, ancient organic remains like pine and pistacia resins, birch bark tar, beeswax, and plant oils as far from bronze and iron age periods were directly analyzed. The advantage of this technique is that the required amount of sample is less and the sample preparation is minimized.[6]
Both direct insertion - MS and gas chromatography - MS were used and compared in a study of characterization of the organic material present as coatings in Roman and Egyptian amphorae can be taken as an example of archaeological resinous materials. From this study, it reveals that, the direct insertion procedure seems to be a fast, straightforward and an unique tool which is suitable for screening of organic archaeological materials which can reveal information about the major constituents within the sample. This method provides information on the degree of oxidation and the class of materials present. As a drawback of this method, less abundant components of the sample may not be identified.[7]
Characterization of synthetic carbon clusters[edit]
Another application of direct insertion EI - MS is the characterization of novel synthetic carbon clusters isolated in the solid phase. These crystalline materials enclose the C60 and C70 in the ratio of 37: 1. In this investigation, it has been shown that the synthetic C60 molecule is remarkably stable and that it retains the aromatic character.[8]
Gas Chromatography EI - MS[edit]
From all of the applications used in EI - MS, Gas Chromatography (GC) is the widely used method for the sample insertion. It is because GC can be incorporated for the separation of mixtures of thermally stable and volatile gases which are in perfect match with the electron ionization conditions.
Analysis of archaeologic materials[edit]
The GC - EI - MS was used for the study and characterization of the organic material present as coatings in Roman and Egyptian amphorae which reveals that, this procedure was more effective to identify the sample composition at the molecular level. From this analysis, scientists found out the material used to coat the amphorae for waterproofing purpose, was not a resin in its native state, but should have been imported from some where else. As the disadvantage of this method, long analysis time and requirement of wet chemical pre-treatment can be mentioned.[7]
Environmental Analysis[edit]
The GC - EI - MS has been successfully used for the determination of pesticide residues in fresh food (vegetables) by a single injection analysis. In this analysis, they have identified 81 multi - class pesticide residues in vegetables. For this analysis, the pesticides were extracted to dichloromethane and further analyzed using gas chromatography – tandem mass spectrometry (GC – MS – MS). The optimum ionization method can be identified as EI or Chemical Ionization (CI) for this single injection of the extract. This method is fast, simple and cost effective since high numbers of pesticides can be determined by GC with one single injection, reducing the total time for the analysis considerably.[9]
Analysis of Biological fluids[edit]
The GC - EI - MS can be incorporated for the analysis of biological fluids for several applications. As an example, this has been used for the determination of thirteen synthetic pyrethroid insecticide molecules and their stereo isomers in entire blood which was investigated using a new rapid and sensitive electron ionization - gas chromatography – mass spectrometry method in selective ion monitoring mode (SIM) by a single injection of the sample. All the pyrethroid residues were separated by using a GC - MS which was operated in electron ionization mode and quantified in selective ion monitoring mode. Even though, the detection of specific residues in blood is a difficult task due to the very low concentration (because as soon as they enter in to the body most of the chemicals may get excreted), this method can detect the residues of different pyrethroids down to the level 0.05–2 ng / ml. The detection of this insecticide in blood is very important since ultra small quantities present in the body is enough to induce a negative impact on human health, especially in children. This method can be mentioned as a very simple, rapid technique and therefore can be adopted without any matrix interferences. Also this is a good method, since the selective ion monitoring mode provides the detection sensitivity up to 0.05 ng/ml.[10] Another application is in the protein turnover studies using GC - EI - MS. In this method,measuring very low levels of enrichment of d,-phenylalanine which can be used to determine the enrichment of amino acid incorporated into tissue protein during the studies of protein synthesis in humans.This method is very efficient since both free and protein - bound d - phenylalanine can be measured using the same mass spectrometer, small amount of protein is needed (about 1 mg) and also because of high precision.[11]
Forensic Applications[edit]
The GC - EI MS is also can be incorporated in forensic practices. As an example, the analysis of five local anesthetics in blood using headspace solid - phase microextraction (HS - SPME) and gas chromatography – mass spectrometry – electron impact ionization selected ion monitoring (GC – MS – EI - SIM) can be pointed out. For the regional treatments, most of the time local anesthesia is widely used even though, these drugs can make occasional medical accidents. Due to this reason, an accurate, simple and rapid method for the analysis of local anesthetics is required. This requirements can be fulfilled using this technique, since the analysis time was 65 minutes and the requirement of small sample amount llike 0.2 g.[12] Another application in forensic practice is the determination of date - rape drugs (DRDs) in urine.These drugs are used in the cases of drugging the victims and raping or robbing them while under the influence of the drug. The analysis of these drugs are difficult due to the low concentrations in the body fluids and a long time delay between the event and clinical examination. However, using GC - EI - MS allows a simple, sensitive and robust method for the identification, detection and quantification of 128 compounds of DRDs in urine.[13]
Liquid Chromatography EI - MS[edit]
Two recent approaches for coupling capillary scale liquid chromatography - electron ionization mass spectrometry (LC - EI - MS), can be incorporated for the analysis of various samples. These two different kinds of interfaces are capillary - scale EI - based LC/MS interface and direct - EI interface. In the capillary EI, the nebulizer has been optimized to have a good linearity and sensitivity. The direct - EI interface is a miniaturized interface for nano - and micro - HPLC, in which the interfacing process takes place into a suitably modified ion source. A superior sensitivity, linearity, and reproducibility can be obtained because of the elution from the column is completely transferred in to the ion source. Using these two interfaces, electron ionization can be successfully incorporated for the analysis of small and medium sized molecules with various polarities. Using these interfaces, most common applications in LC - MS are environmental applications like gradient separations of three pesticides carbaryl, propanil, and chlorpropham using a reversed phase and pharmaceutical applications such as separation of four anti-inflammatory drugs diphenyldramine, amitryptyline, naproxen, and ibuprofen.[14]
Another method to categorize the applications of electron ionization is based on the separation technique which is used in mass spectroscopy. According to this category, most of the time applications can be found in Time of Flight (TOF) or orthogonal TOF mass spectrometry (OA - TOF MS), Fourier Transform Ion Cyclotron Resonance Mass Spectrometry (FT - ICR MS) and quadruple or ion trap mass spectrometry.
TOF or OA- TOF EI - MS[edit]
The electron ionization Time of Flight mass spectroscopy (EI-TOF MS) is performed significantly, suited for analytical and basic chemical physics studies. The investigations about the positive ions by EI-TOF MS is used to find the many ionization potentials of molecules and radicals, as well as accepted bond dissociation energies for ions and neutral molecules. Another importance of this method is to study about negative ion chemistry and physics. Most of the properties of negative ions have been discovered using this technique. As an example, autodetachment lifetimes, metastable dissociation, Rydberg electron transfer reactions and field detachment, SF6 Scavenger method for detecting temporary negative ion states, and many others .In this method, field free ionization region allows for high precision in the electron energy and also high electron energy resolution. Using the electric fields down the ion flight tube helps to study of the autodetachment and metastable decomposition as well as field detachment of weakly bound negative ions.[15]
The first description of an EI oa-TOFMS was in 1989 and by using "orthogonal-acceleration" with the EI ion source ,the resolving power and sensitivity can be increased. One of the key advantage of oa-TOFMS with EI sources is for deployment with gas chromatographic (GC) inlet systems. Due to this reason, chromatographic separation of volatile organic compounds can now proceed at high speed.[16]
FT- ICR EI- MS[edit]
FT- ICR EI - MS can be used for analysis of three vacuum gas oil distillation fractions in 295-319 °C, 319-456 °C, and 456-543 °C. In this method, EI at 10 eV allowed soft ionization of aromatic compounds in the vacuum gas oil range. The compositional variations at the molecular level was determined with out any doubt from the elemental composition assignment. Ultra high resolving power, small sample size,high reproducibility and mass accuracy (<0.4ppm) can be mentioned as the special features in this method. The major product was aromatic hydrocarbons in all three samples. In addition, many sulfur-, nitrogen-, and oxygen-containing compounds were directly observed.When the concentration of this heteroatomic species increase with the boiling point. Using data analysis it gave the information about compound types (rings plus double bonds), their carbon number distributions for hydrocarbon and heteroatomic compounds in the distillation fractions, increasing average molecular weight (or carbon number distribution) and aromaticity with increasing boiling temperature of the petroleum fractions.[17]
Ion Trap EI - MS[edit]
Ion trap EI MS can be incorporated for the identification and quantitation of nonylphenol polyethoxylate (NPEO) residues and their degradation products such as nonylphenol polyethoxy carboxylates and carboxyalkylphenol ethoxy carboxylates, in the samples of river water and sewage effluent. Form this research, they have found out that the ion trap GC- MS is a reliable and convenient analytical approach with variety of ionization methods including EI, for the determination of target compounds in environmental samples.[18]
Advantages/DisadvantagesThere are several advantages and also disadvantages by using EI as the ionization method in mass spectrometry. These are listed below.[1][edit]
| Advantages | Disadvantages |
|---|---|
| Simple | Molecule must be volatile |
| Sensitive | molecule must be thermally stable |
| Fragmentation aids Identification of molecules | Extensive fragmentation- can't interpret data |
| Library-searchable fingerprint spectra | Useful mass range is low (<1000 Da) |
See Also[edit]
References[edit]
- ^ a b c d e Dass, Chhabil. Fundamentals of Contemporary Mass Spectrometry - Dass - Wiley Online Library. doi:10.1002/0470118490.
- ^ Dempster, A. J. (1918-04-01). "A new Method of Positive Ray Analysis". Physical Review. 11 (4): 316–325. doi:10.1103/PhysRev.11.316.
- ^ Dempster, A. J. (1921-01-01). "Positive Ray Analysis of Lithium and Magnesium". Physical Review. 18 (6): 415–422. doi:10.1103/PhysRev.18.415.
- ^ R. Davis, M. Frearson, (1987). Mass Spectrometry – Analytical Chemistry by Open Learning, John Wiley & Sons, London.
- ^ K. Robinson et al. Undergraduate Instrumental Analysis, 6th ed. Marcel Drekker, New York, 2005.
- ^ Regert, Martine; Rolando, Christian (2002-02-02). "Identification of Archaeological Adhesives Using Direct Inlet Electron Ionization Mass Spectrometry". Analytical Chemistry. 74 (5): 965–975. doi:10.1021/ac0155862.
- ^ a b Colombini, Maria Perla; Modugno, Francesca; Ribechini, Erika (2005-05-01). "Direct exposure electron ionization mass spectrometry and gas chromatography/mass spectrometry techniques to study organic coatings on archaeological amphorae". Journal of Mass Spectrometry. 40 (5): 675–687. doi:10.1002/jms.841. ISSN 1096-9888.
- ^ Luffer, Debra R.; Schram, Karl H. (1990-12-01). "Electron ionization mass spectrometry of synthetic C60". Rapid Communications in Mass Spectrometry. 4 (12): 552–556. doi:10.1002/rcm.1290041218. ISSN 1097-0231.
- ^ Arrebola, F. J.; Martı́nez Vidal, J. L.; Mateu-Sánchez, M.; Álvarez-Castellón, F. J. (2003-05-19). "Determination of 81 multiclass pesticides in fresh foodstuffs by a single injection analysis using gas chromatography–chemical ionization and electron ionization tandem mass spectrometry". Analytica Chimica Acta. 484 (2): 167–180. doi:10.1016/S0003-2670(03)00332-5.
- ^ Ramesh, Atmakuru; Ravi, Perumal Elumalai (2004-04-05). "Electron ionization gas chromatography–mass spectrometric determination of residues of thirteen pyrethroid insecticides in whole blood". Journal of Chromatography B. 802 (2): 371–376. doi:10.1016/j.jchromb.2003.12.016.
- ^ Calder, A. G.; Anderson, S. E.; Grant, I.; McNurlan, M. A.; Garlick, P. J. (1992-07-01). "The determination of low d5-phenylalanine enrichment (0.002–0.09 atom percent excess), after conversion to phenylethylamine, in relation to protein turnover studies by gass chromatography/electron ionization mass spectrometry". Rapid Communications in Mass Spectrometry. 6 (7): 421–424. doi:10.1002/rcm.1290060704. ISSN 1097-0231.
- ^ Watanabe, Tomohiko; Namera, Akira; Yashiki, Mikio; Iwasaki, Yasumasa; Kojima, Tohru (1998-05-29). "Simple analysis of local anaesthetics in human blood using headspace solid-phase microextraction and gas chromatography–mass spectrometry–electron impact ionization selected ion monitoring". Journal of Chromatography B: Biomedical Sciences and Applications. 709 (2): 225–232. doi:10.1016/S0378-4347(98)00081-4.
- ^ Adamowicz, Piotr; Kała, Maria. "Simultaneous screening for and determination of 128 date-rape drugs in urine by gas chromatography–electron ionization-mass spectrometry". Forensic Science International. 198 (1–3): 39–45. doi:10.1016/j.forsciint.2010.02.012.
- ^ Cappiello, Achille; Famiglini, Giorgio; Mangani, Filippo; Palma, Pierangela (2001-01-01). "New trends in the application of electron ionization to liquid chromatography—mass spectrometry interfacing". Mass Spectrometry Reviews. 20 (2): 88–104. doi:10.1002/mas.1004. ISSN 1098-2787.
- ^ Mirsaleh-Kohan, Nasrin; Robertson, Wesley D.; Compton, Robert N. (2008-05-01). "Electron ionization time-of-flight mass spectrometry: Historical review and current applications". Mass Spectrometry Reviews. 27 (3): 237–285. doi:10.1002/mas.20162. ISSN 1098-2787.
- ^ Guilhaus, M.; Selby, D.; Mlynski, V. (2000-01-01). "Orthogonal acceleration time-of-flight mass spectrometry". Mass Spectrometry Reviews. 19 (2): 65–107. doi:10.1002/(SICI)1098-2787(2000)19:23.0.CO;2-E. ISSN 1098-2787.
- ^ Fu, Jinmei; Kim, Sunghwan; Rodgers, Ryan P.; Hendrickson, Christopher L.; Marshall, Alan G.; Qian, Kuangnan (2006-02-08). "Nonpolar Compositional Analysis of Vacuum Gas Oil Distillation Fractions by Electron Ionization Fourier Transform Ion Cyclotron Resonance Mass Spectrometry". Energy & Fuels. 20 (2): 661–667. doi:10.1021/ef0503515.
- ^ Ding, Wang-Hsien; Tzing, Shin-Haw (1998-10-16). "Analysis of nonylphenol polyethoxylates and their degradation products in river water and sewage effluent by gas chromatography–ion trap (tandem) mass spectrometry with electron impact and chemical ionization". Journal of Chromatography A. 824 (1): 79–90. doi:10.1016/S0021-9673(98)00593-7.
- ^ Lakowicz, Joseph R. (2007-12-05). Principles of Fluorescence Spectroscopy. Springer Science & Business Media. ISBN 9780387463124.
Notes[edit]
- Edmond de Hoffman; Vincent Stroobant (2001). Mass Spectrometry: Principles and Applications (2nd ed.). John Wiley and Sons. ISBN 0-471-48566-7.
- Stephen J. Schrader (2001). Interpretation of Electron Ionization Data: The Odd Book. Not Avail. ISBN 0-9660813-6-6.
- Peterkops, Raimonds (1977). Theory of ionization of atoms by electron impact. Boulder, Colo: Colorado Associated University Press. ISBN 0-87081-105-3.
- Electron impact ionization. Berlin: Springer-Verlag. 1985. ISBN 0-387-81778-6.
External links[edit]
- NIST Chemistry WebBook
- Mass Spectrometry. Michigan State University.
Category:Ion source
Category:Mass spectrometry
Category:Scientific techniques


![{\displaystyle I^{+}=\beta Q_{i}L[N]I_{e}}](https://wikimedia.org/api/rest_v1/media/math/render/svg/fa6a0fc6f9d9f314fd864e8df524cfdf668ee502)