User:Mr. Ibrahem/Pseudoephedrine
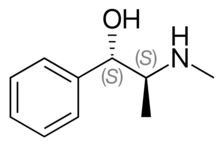 | |
 | |
| Clinical data | |
|---|---|
| Pronunciation | /ˌsuːdoʊ.ɪˈfɛdrɪn, -ˈɛfɪdriːn/ |
| Trade names | Afrinol, Sudafed, Sinutab, others |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a682619 |
| License data | |
| Pregnancy category |
|
| Routes of administration | By mouth |
| Drug class | Sympathomimetic (alpha and beta adrenergic agonist)[1][2] |
| Legal status | |
| Legal status |
|
| Pharmacokinetic data | |
| Bioavailability | ~100%[3] |
| Metabolism | 10–30% liver |
| Elimination half-life | 4.3–8 hours[3] |
| Excretion | 43–96% kidney[3] |
| Identifiers | |
| |
| Chemical and physical data | |
| Formula | C10H15NO |
| Molar mass | 165.23 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| (verify) | |
Pseudoephedrine (PSE) is a medication used for congestion of the nose such as may occur from hay fever or the common cold.[1] It may also be used to prevent pressure related ear problems due to eustachian tube obstruction.[1] It has not been found to be useful for sinusitis.[1] Use is not recommended in children less than six.[2] It is sold both by itself and over-the-counter in combination with other active ingredients such as antihistamines, guaifenesin, dextromethorphan, paracetamol (acetaminophen), or NSAIDs.[1][2] It is taken by mouth.[1]
Common side effects include trouble sleeping, palpitations, headache, and dizziness.[1] Other concerns include abuse.[1] Use in early pregnancy is associated with harm to the baby well use during the early part of breastfeeding may reduce milk output.[2] It is a sympathomimetic and alpha and beta adrenergic agonist.[1][2]
Pseudoephedrine was isolated in 1889, by the German chemists Ladenburg and Oelschlägel, from Ephedra vulgaris at the Merck pharmaceutical company.[4][5] Plants that contain the medication; however, have been used in Chinese medicine for 5,000 years.[5] At higher doses it is used as a wakefulness-promoting agent and to enhance athletic performance.[6] Such use, has at various times, not been permitted by the International Olympic Committee.[6] Pseudoephedrine has also been used to illegally manufacture methamphetamines.[1] In the United Kingdom 24 tabs of 60 mg costs the NHS about 2 pounds.[2]
References[edit]
- ^ a b c d e f g h i j k l "Pseudoephedrine Monograph for Professionals". Drugs.com. Archived from the original on 9 March 2021. Retrieved 5 October 2020.
- ^ a b c d e f BNF 79. London: Pharmaceutical Press. March 2020. p. 1239. ISBN 978-0857113658.
- ^ a b c Laurence L Brunton, ed. (2006). Goodman & Gilman's The Pharmacological Basis of Therapeutics (11th ed.). New York: McGraw-Hill Medical Publishing Division. ISBN 0-07-142280-3.
- ^ Ladenburg, A.; Oelschlägel, C. (1889). "Ueber das "Pseudo-Ephedrin"" [On pseudo-ephedrine]. Berichte der Deutschen Chemischen Gesellschaft (in German). 22 (2): 1823–1827. doi:10.1002/cber.18890220225. Archived from the original on 2021-03-08. Retrieved 2019-05-19.
- ^ a b Farmer, Steven (2017). Strange Chemistry: The Stories Your Chemistry Teacher Wouldn't Tell You. John Wiley & Sons. p. 294. ISBN 978-1-119-26529-0. Archived from the original on 29 August 2021. Retrieved 6 October 2020.
- ^ a b Trinh, KV; Kim, J; Ritsma, A (15 November 2015). "Effect of Pseudoephedrine in Sport: a Systemic Review". BMJ Open Sport & Exercise Medicine. 1 (1): e000066. doi:10.1136/bmjsem-2015-000066. PMC 5117033. PMID 27900142.
