User:Mr. Ibrahem/Gefitinib
 | |
 | |
| Clinical data | |
|---|---|
| Pronunciation | /ɡɛˈfɪtɪnɪb/ |
| Trade names | Iressa, others |
| Other names | ZD1839 |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a607002 |
| License data | |
| Pregnancy category |
|
| Routes of administration | By mouth |
| Drug class | Tyrosine kinase inhibitor (EGFR inhibitor)[1] |
| Legal status | |
| Legal status | |
| Pharmacokinetic data | |
| Bioavailability | 59% (by mouth) |
| Protein binding | 90% |
| Metabolism | Liver (mainly CYP3A4) |
| Elimination half-life | 6–49 hours |
| Excretion | Feces |
| Identifiers | |
| |
| Chemical and physical data | |
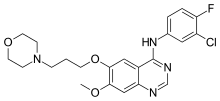
| Formula | C22H24ClFN4O3 |
| Molar mass | 446.91 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| (verify) | |
Gefitinib, sold under the brand name Iressa, is a medication used to treat non-small cell lung cancer (NSCLC).[1] Specifically it is used in cases which have certain mutations of the epidermal growth factor receptor.[2] It is taken by mouth.[1]
Common side effects include rash, diarrhea, nausea, fever, mouth inflammation, eye problems, liver problems, and kidney problems.[1] Other side effects may include interstitial lung disease and infertility.[1] Use in pregnancy may harm the baby.[1] It is a tyrosine kinase inhibitor and EGFR inhibitor.[1]
Gefitinib was approved for medical use in the United States in 2003 and Europe in 2009.[1][3] It is on the World Health Organization's List of Essential Medicines as an alternative to erlotinib.[4] It is available as a generic medication.[2] In the United Kingdom a month costs the NHS about £2,200 as of 2021.[2] This amount in the United States costs about 8,100 USD.[5]
References[edit]
- ^ a b c d e f g h i j "Gefitinib Monograph for Professionals". Drugs.com. Archived from the original on 5 November 2021. Retrieved 3 December 2021.
- ^ a b c d BNF 81: March-September 2021. BMJ Group and the Pharmaceutical Press. 2021. p. 1027. ISBN 978-0857114105.
- ^ "Iressa". Archived from the original on 5 November 2021. Retrieved 3 December 2021.
- ^ World Health Organization (2021). World Health Organization model list of essential medicines: 22nd list (2021). Geneva: World Health Organization. hdl:10665/345533. WHO/MHP/HPS/EML/2021.02.
- ^ "Iressa Prices, Coupons & Patient Assistance Programs". Drugs.com. Archived from the original on 17 May 2016. Retrieved 3 December 2021.
