User:Mr. Ibrahem/Flibanserin
 | |
 | |
| Clinical data | |
|---|---|
| Trade names | Addyi |
| AHFS/Drugs.com | Monograph |
| License data |
|
| Routes of administration | By mouth |
| Legal status | |
| Legal status | |
| Pharmacokinetic data | |
| Bioavailability | 33%[1] |
| Protein binding | ~98% |
| Metabolism | Extensive by liver (mainly by CYP3A4 and CYP2C19) |
| Elimination half-life | ~11 hours |
| Excretion | Biliary (51%), kidney (44%) |
| Identifiers | |
| |
| Chemical and physical data | |
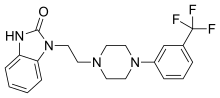
| Formula | C20H21F3N4O |
| Molar mass | 390.410 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| | |
Flibanserin, sold under the brand name Addyi, is a medication used to treat women with hypoactive sexual desire disorder (HSDD).[2] It increases the number of satisfying sexual events per month by about one half from a starting point of about two to three.[3][4] The certainty of this estimate is low.[3] It is taken by mouth.[2]
Common side effects include dizziness, sleepiness, nausea, and tiredness.[3] Other side effects may include low blood pressure.[2] Use with alcohol or in those with liver problems is not recommended.[2] There are concerns that use in pregnancy may harm the baby.[5] It is a 5-HT1A receptor activator and 5-HT2A receptor inhibitor.[2]
Flibanserin was approved for medical use in the United States in 2015 and Canada in 2018.[2][6] As of 2021 it is not approved in the United Kingdom.[7] In the United States it costs about 490 USD per month as of 2021.[8]
References[edit]
- ^ a b "Addyi- flibanserin tablet, film coated". DailyMed. 10 October 2019. Archived from the original on 20 October 2020. Retrieved 20 October 2020.
- ^ a b c d e f g h i "Flibanserin Monograph for Professionals". Drugs.com. Retrieved 11 December 2021.
- ^ a b c d Jaspers, Loes; Feys, Frederik; Bramer, Wichor M.; Franco, Oscar H.; Leusink, Peter; Laan, Ellen T. M. (29 February 2016). "Efficacy and Safety of Flibanserin for the Treatment of Hypoactive Sexual Desire Disorder in Women". JAMA Internal Medicine. 176 (4): 453–62. doi:10.1001/jamainternmed.2015.8565. PMID 26927498.
- ^ "Joint Meeting of the Bone, Reproductive and Urologic Drugs Advisory Committee (BRUDAC) and the Drug Safety and Risk Management (DSaRM) Advisory Committee" (PDF). June 4, 2015. Archived (PDF) from the original on 5 June 2015. Retrieved 5 June 2015.
- ^ "Flibanserin (Addyi) Use During Pregnancy". Drugs.com. Archived from the original on 4 December 2020. Retrieved 11 December 2021.
- ^ Government of Canada, Health Canada (25 April 2012). "Drug Product Database Online Query". health-products.canada.ca. Archived from the original on 11 December 2021. Retrieved 11 December 2021.
- ^ Nast, Condé (6 August 2021). "Is there a female equivalent of Viagra? And if so, does it actually work?". Glamour UK. Archived from the original on 11 December 2021. Retrieved 11 December 2021.
- ^ "Flibanserin Prices, Coupons & Savings Tips - GoodRx". GoodRx. Archived from the original on 11 May 2016. Retrieved 11 December 2021.
