User:Mr. Ibrahem/Cefaclor
 | |
| Clinical data | |
|---|---|
| Trade names | Distaclor,[1] Medacef,[2] Keflor, others |
| Other names | Cephaclor |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a682729 |
| Pregnancy category |
|
| Routes of administration | By mouth |
| Drug class | Antibiotic (2nd generation cephalosporin)[3] |
| Legal status | |
| Legal status |
|
| Pharmacokinetic data | |
| Bioavailability | Well absorbed, independent of food intake |
| Metabolism | 15% to 40% |
| Elimination half-life | 0.6 to 0.9 hours |
| Excretion | Kidney |
| Identifiers | |
| |
| Chemical and physical data | |
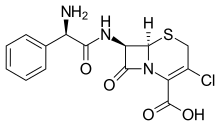
| Formula | C15H14ClN3O4S |
| Molar mass | 367.80 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| | |
Cefaclor, sold under the brand name Ceclor among others, is an antibiotic used to treat bacterial infections such as pneumonia, middle ear infections, strep throat, cellulitis, and urinary tract infections.[4] It is taken by mouth with food.[1] It may be used in children as young as a month old.[1]
Common side effects include rash, diarrhea, vaginitis, nausea, and headache.[4] Other side effects may include anxiety, low red blood cells, joint pains, allergic reactions, jaundice, and swollen glands.[1] It is not known to cause harm in pregnancy and may be used when breastfeeding.[1] It is a second-generation cephalosporin.[3]
Cefaclor was patented in 1975 and approved for medical use in 1979.[5][6] In the United Kingdom, a course of treatment generally costs the NHS less than £10, as of 2021.[1] This amount in the United States costs about 22 USD.[7]
References[edit]
- ^ a b c d e f "5. Infection". British National Formulary (BNF) (82 ed.). London: BMJ Group and the Pharmaceutical Press. September 2021 – March 2022. pp. 559–560. ISBN 978-0-85711-413-6.
{{cite book}}: CS1 maint: date format (link) - ^ "A - Z Drug List from Drugs.com: Cefaclor". Drugs.com. American Society of Health-System Pharmacists. Archived from the original on 17 November 2021. Retrieved 27 November 2021.
- ^ a b Beauduy, Camille E.; Winston, Lisa G. (2020). "43. Beta-lactam and other cell wall - & membrane - active antibiotics". In Katzung, Bertram G.; Trevor, Anthony J. (eds.). Basic and Clinical Pharmacology (15th ed.). New York: McGraw-Hill. pp. 832–834. ISBN 978-1-260-45231-0. Archived from the original on 2021-10-10. Retrieved 2021-11-27.
- ^ a b c d "Cefaclor Monograph for Professionals". Drugs.com. Archived from the original on 26 February 2021. Retrieved 30 December 2021.
- ^ Nard, Craig Allen (2020). "7. Enforcing patent rights". The Law of Patents. New York: Wolters Kluwer. p. 694. ISBN 978-1-5438-1368-5. Archived from the original on 2021-12-11. Retrieved 2021-12-01.
- ^ Fischer, Jnos; Ganellin, C. Robin (2006). Analogue-based Drug Discovery. John Wiley & Sons. p. 493. ISBN 9783527607495. Archived from the original on 2017-09-10. Retrieved 2020-12-31.
- ^ "Cefaclor Prices, Coupons & Savings Tips - GoodRx". GoodRx. Retrieved 30 December 2021.
