User:Hjq00/sandbox
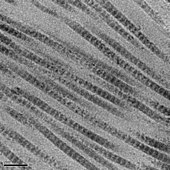
The tropocollagen subunits spontaneously self-assemble, with regularly staggered ends, into even larger arrays in the extracellular spaces of tissues.[1][2] Additional assembly of fibrils is guided by fibroblasts, which deposit fully formed fibrils from fibripositors. In the fibrillar collagens, molecules are staggered to adjacent molecules by approximately 64-67 nm, although up to a 10 nm distribution is intrinsic to collagen fibrils. This variation is hypothesized to arise from differing mechanical stressors, expression of other collagen types nearby, post-translational modifications, and/or fibrillar tilting and supercoiling. [3] The unit is referred to as 'D' and is also dependent on the tissue and hydration state of the aggregate; the longitudinal distance between repeating molecules is 40 nm[4]. Each D-period repeat of the microfibril consists of an "overlap" zone and a "gap" zone. The overlap zone consists of five collagen molecules aligned laterally and the gap zone consists of only four collagen molecules in cross-section[4]. These overlap and gap regions are retained as microfibrils assemble into fibrils, and are thus viewable using electron microscopy. The triple helical tropocollagens in the microfibrils are arranged in a quasihexagonal packing pattern.[5][6] The D-period plays a significant role in the mechanical properties of fibrils and cell-collagen crosstalk, and has also been correlated with various pathologies. Studies employing atomic force microscopy (AFM) have found changes in the D-band length assoicated with aging, diabetes, disc degeneration, and numerous genetic disorders[7]
- ^ Hulmes, D. J. (2002). "Building collagen molecules, fibrils, and suprafibrillar structures". J Struct Biol. 137 (1–2): 2–10. doi:10.1006/jsbi.2002.4450. PMID 12064927.
- ^ Hulmes, D. J. (1992). "The collagen superfamily – diverse structures and assemblies". Essays Biochem. 27: 49–67. PMID 1425603.
- ^ Fang, Ming; Holl, Mark (21 August 2013). "Variation in type I collagen fibril nanomorphology: the significance and origin". BoneKEy Reports. doi:10.1038/bonekey.2013.128.
- ^ a b Zhao, Chenxi; Xiao, Yuelong; Ling, Shengjie; Pei, Ying; Ren, Jing (2 September 2021). "Structure of Collagen". Fibrous Proteins. 2347: 17–25. doi:10.1007/978-1-0716-1574-4_2.
- ^ Orgel, Joseph; Irving, Thomas; Miller, Andrew; Wess, Tim (13 June 2006). "Microfibrillar structure of type I collagen in situ". PNAS. 103 (24). doi:10.1073/pnas.0502718103.
- ^ Hulmes, David; Miller, Andrew (20 December 1979). "Quasi-hexagonal molecular packing in collagen fibrils". Nature: 878-880. doi:10.1038/282878a0.
- ^ Stylianou, Andreas (21 February 2022). "Assessing Collagen D-Band Periodicity with Atomic Force Microscopy". Materials. 15 (4). doi:10.3390/ma15041608.
{{cite journal}}: CS1 maint: unflagged free DOI (link)

