User:DSchlink15/ABCG2
| This is the sandbox page where you will draft your initial Wikipedia contribution.
If you're starting a new article, you can develop it here until it's ready to go live. If you're working on improvements to an existing article, copy only one section at a time of the article to this sandbox to work on, and be sure to use an edit summary linking to the article you copied from. Do not copy over the entire article. You can find additional instructions here. Remember to save your work regularly using the "Publish page" button. (It just means 'save'; it will still be in the sandbox.) You can add bold formatting to your additions to differentiate them from existing content. |
ABCG2 draft[edit]
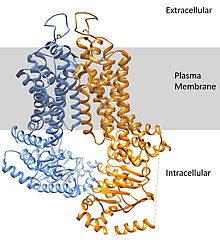
ATP-binding cassette super-family G member 2 (ABCG2) is a protein that in humans is encoded by the ABCG2 gene. ABCG2 has also been designated as CDw338 (cluster of differentiation w338). ABCG2 is a translocation protein used to actively pump drugs and other compounds against their concentration gradient using the bonding and hydrolysis of ATP as the energy source. 3
ABCG2 forms into a homodimer to assume its active transport conformation. The dimer weighs approximately 144 kDa. The expression of this transport protein is highly conserved throughout the animal kingdom, pointing to its importance. 4
Substrate binding with compounds occurs in the large central cavity. ABCG2 can bind to a broad range of compounds but binds strongest to flat, polycyclic chemicals with lots of hydrophobic character.3
Function[edit]
The membrane-associated protein encoded by this gene is included in the superfamily of ATP-binding cassette (ABC) transporters. ABC proteins transport various molecules across extra- and intra-cellular membranes. The active transport of chemicals requires a source of energy to catalyze the conformational changes the protein undergoes. The nucleotide-binding domains (NBDs) found towards the N-terminus allow binding to ATP molecules. The NBD and the transmembrane domain (TMD) are the most conserved region of the transporter in various animal groups, highlighting the importance of these regions for overall protein function.4 Additionally, many ABC transporters have conserved NBD regions showing the strict conformation needed to bind ATP molecules.3 ABC genes are divided into seven distinct subfamilies (ABC1, MDR/TAP, MRP, ALD, OABP, GCN20, White). This protein is a member of the White subfamily. Alternatively referred to as the breast cancer resistance protein (BCRP), this protein functions as a xenobiotic transporter which may play a role in multi-drug resistance to chemotherapeutic agents including mitoxantrone and camptothecin analogues. Early observations of significant ABCG2-mediated resistance to anthracyclines were subsequently attributed mutations encountered in vitro but not in nature or the clinic. Significant expression of this protein has been observed in the placenta, and it has been shown to have a role in protecting the fetus from xenobiotics in the maternal circulation.
The transporter has been shown to play protective roles in blocking absorption at the apical membrane of the intestine, and at the blood-testis barrier, the blood–brain barrier, and the membranes of hematopoietic progenitor and other stem cells. At the apical membranes of the liver and kidney, it enhances excretion of xenobiotics. In the lactating mammary gland, it has a role on excreting vitamins such as riboflavin and biotin into milk. Xenobiotic toxins compete for the substrate binding domain of ABCG2 potentially causing higher toxin levels in breast milk than intended.4 In the kidney and gastrointestinal tract, it has a role in urate excretion.
The protein also carries the Jr(a) antigen, which defines the Junior blood group system.
Transport Mechanism[edit]
Inhibition[edit]
It is inhibited by some calcium channel blockers such as amlodipine, felodipine and nifedipine. The fungal toxin fumitremorgin C (FTC) inhibits the protein but has neurotoxic side effects. A synthetic tetracyclic analog of FTC called Ko-143 inhibits ABCG2. 2
References[edit]
- Khunweeraphong, N.; Szöllősi, D.; Stockner, T.; Kuchler, K. The ABCG2 Multidrug Transporter Is a Pump Gated by a Valve and an Extracellular Lid. Nature Communications 2019, 10 (1), 5433. https://doi.org/10.1038/s41467-019-13302-2.
- Jackson, S. M.; Manolaridis, I.; Kowal, J.; Zechner, M.; Taylor, N. M. I.; Bause, M.; Bauer, S.; Bartholomaeus, R.; Bernhardt, G.; Koenig, B.; Buschauer, A.; Stahlberg, H.; Altmann, K.-H.; Locher, K. P. Structural Basis of Small-Molecule Inhibition of Human Multidrug Transporter ABCG2. Nature Structural Molecular Biology 2018, 25 (4), 333–340. https://doi.org/10.1038/s41594-018-0049-1.
- Taylor, N. M. I.; Manolaridis, I.; Jackson, S. M.; Kowal, J.; Stahlberg, H.; Locher, K. P. Structure of the Human Multidrug Transporter ABCG2. Nature 2017, 546 (7659), 504–509. https://doi.org/10.1038/nature22345.
- Robey, R. W.; To, K. K. K.; Polgar, O.; Dohse, M.; Fetsch, P.; Dean, M.; Bates, S. E. ABCG2: A Perspective. Advanced Drug Delivery Reviews 2009, 61 (1), 3–13. https://doi.org/10.1016/j.addr.2008.11.003.
