User:CelsoGomez/sandbox
In the mitochondrion, the matrix is the space within the inner membrane.The word "matrix" stems from the fact that this space is viscous, compared to the relatively aqueous cytoplasm. The matrix facilitates reactions responsible for the production of ATP, such as the citric acid cycle, oxidative phosphorylation, oxidation of pyruvate and the beta oxidation of fatty acids.[1]
The mitochondrial matrix contains the mitochondria's DNA, ribosomes, soluble enzymes that catalyze the oxidation of pyruvate, small organic molecules, nucleotide cofactors, and inorganic ions.[1] The compositon of the matrix based on its structures and contents produce an environment that allows the anabolic and catabolic pathways to proceed favorably. The electron transport chain and enzymes in the matrix play a large role in the citric acid cycle and oxidative phosphorylation. The citric acid cycle produces NADH and FADH2 through oxidation that will be reduced in oxidative phosphorylation to produce ATP.[2][3]
The cytosolic, intermembrane space, compartment has a water content of 3.8 μl/mg protein, while the mitochondrial matrix 0.8 μl/mg protein.[4] It is not known how mitochondria maintain osmotic balance across the inner mitochondrial membrane, although the membrane contains aquaporins that are believed to be conduits for regulated water transport. Mitochondrial matrix has a pH of about 7.8.[5] Mitochondrial DNA was discovered by Nash and Margit in 1963. One to many double stranded mainly circular DNA is present in mitochondrial matrix. Mitochondrial DNA is 1% of total DNA of a cell. It is rich in Guanine and Cytosine content. Mitochondria of mammals have 55s ribosomes.
Composition[edit]
The matrix is bound by the inner membrane which results in the characteristics that makes facilitation of anabolic and catabolic pathways possible. The matrix contains 2/3 of the total proteins found in the mitochondria.[6] The inner membrane is a phospholipid bilayer that contains the electron transport chain. The electron transport chain consists of four protein complexes and ATP synthase. These protein complexes are found in the many cristae seen throughout the inner membrane. The electron transport chain coupled with oxidative phosphorylation is responsible for establishing a pH and electrochemical gradient that facilitates the production of ATP. The gradient also provides control of the concentration of ions such as Ca2+ driven by the mitochondrial membrane potential.[1] The inner membrane phospholipid bilayer contains cardiolipin which makes the bilayer semi permeable. The membrane only allows nonpolar molecules such as CO2 and O2 and small non charged polar molecules such as H2O. Molecules enter and exit the mitochondrial matrix through transport proteins and ion transporters. Molecules are then able to leave the mitochondria through porin.[6] These attributed characteristics allow for control over concentrations of ions and metabolites in order to regulate the citric acid cycle, oxidation of pyruvate, beta oxidation of fatty acids, gluconeogenesis, oxidative phosphorylation, and the rate of ATP production. The matrix is also host to enzymes, ribosomes, tRNA, DNA, intermediates, and cofactors. The mitochondrial matrix provides environmental conditions that facilitate biological pathways due to the composition of the inner membrane and the relationships between the various pathways in the matrix.[7][8]
Facilitated Reactions In the Mitochondrial Matrix[edit]
Acetyl-CoA Production[edit]
Following glycolysis, the citric acid cycle is activated by the production of acetyl-CoA. The oxidation of pyruvate by coenzyme A in the matrix poroduces CO2, acetyl-CoA, and NADH. Beta oxidation of fatty acids serves as an alternate catabolic pathway that produces acetyl-CoA, NADH, and FADH2.[1] The production of acetyl-CoA begins the citric acid cycle while the co-enzymes produced are used in the electron transport chain.[8]
The Citric Acid Cycle[edit]
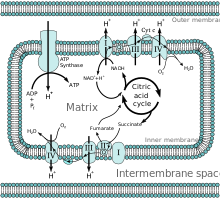
All of the enzymes for the citric acid cycle are in the matrix (e.g. citrate synthase, iso-citrate dehydrogenase, alpha-ketoglutarate dehydrogenase, fumarase, and malate dehydrogenase) except for succinate dehydrogenase which is on the inner membrane and is part of protein complex II in the electron transport chain. This allows the Production of GTP or ATP as NADH is reduced.[2] Regulation through concentration exhibited by the selective permeability of the phospholipid bilayer is demonstrated through ions and intermediates. Ca2+ in the matrix activates pyruvate dehydrogenase, isocitrate dehydrogenase, and alpha-ketoglutarate dehydrogenase which increases the reaction rate in the cycle.[9] Concentration of intermediates in the matrix also increase or decrease the rate of ATP production due to anaplerotic and cataplerotic effects.[2]
Oxidative Phosphorylation and the Electron Transport Chain[edit]
The citric acid cycle produces NADH and FADH2, while glycolysis and beta oxidation produce NADH. These co-enzymes are produced in the matrix or transported in through porin and transport proteins in order to undergo reduction through oxidative phosphorylation.[1] NADH and FADH2 undergo reduction in the electron transport chain by transferring a proton and electron to regenerate NAD+and FAD. Protons are pulled into the intermembrane space by the energy of the electrons going through the electron transport chain. Two electrons are finally accepted by oxygen in the matrix to complete the electron transport chain. The protons return to the mitochondrial matrix through the process of chemiosmosis through the protein ATP synthase.The energy is used in order to rotate ATP synthase which facilitates the passage of a proton, producing ATP. A pH difference in the matrix and intermembrane space creates a gradient by which ATP synthase can pass a proton into the matrix favorably. The proton concentration in the matrix is maintained by reactions of the electron transport chain and creates an electrochemical gradient.[10]
- ^ a b c d Voet, Donald; Voet, Judith; Pratt, Charlotte (2013). Fundamentals of Biochemistry Life at the Molecular Level. New York City: John Wiley & Sons, Inc. pp. 582–584. ISBN 1118129180.
- ^ a b c Stryer, L; Berg, J; Tymoczko, JL (2002). Biochemistry. San Francisco: W.H. Freeman. pp. 509–527, 569–579, 614–616, 638–641, 732–735, 739–748, 770–773. ISBN 0-7167-4684-0.
- ^ Mitchell, Peter; Moyle, Jennifer (1967-01-14). "Chemiosmotic Hypothesis of Oxidative Phosphorylation". Nature. 213 (5072): 137–139. doi:10.1038/213137a0.
- ^ Soboll, S; Scholz, R; Freisl, M; Elbers, R; Heldt, H.W. (1976). Distribution of metabolites between mitochondria and cytosol of perfused liver. New york: Elsevier. pp. 29–40. ISBN 978-0-444-10925-5.
- ^ Porcelli, Anna Maria; Ghelli, Anna; Zanna, Claudia; Pinton, Paolo; Rizzuto, Rosario; Rugolo, Michela (2005-01-28). "pH difference across the outer mitochondrial membrane measured with a green fluorescent protein mutant". Biochemical and Biophysical Research Communications. 326 (4): 799–804. doi:10.1016/j.bbrc.2004.11.105.
- ^ a b Alberts, Bruce; Johnson, Alexander; Lewis, julian; Roberts, Keith; Peters, Walter; Raff, Martin (1994). Molecular Biology of the Cell. New york: Garland Publishing Inc. ISBN 0-8153-3218-1.
- ^ Anderson, S.; Bankier, A. T.; Barrell, B. G.; de Bruijn, M. H. L.; Coulson, A. R.; Drouin, J.; Eperon, I. C.; Nierlich, D. P.; Roe, B. A. (1981-04-09). "Sequence and organization of the human mitochondrial genome". Nature. 290 (5806): 457–465. doi:10.1038/290457a0.
- ^ a b Iuchi, S.; Lin, E. C. C. (1993-07-01). "Adaptation of Escherichia coli to redox environments by gene expression". Molecular Microbiology. 9 (1): 9–15. doi:10.1111/j.1365-2958.1993.tb01664.x. ISSN 1365-2958.
- ^ Denton, Richard M.; Randle, Philip J.; Bridges, Barbara J.; Cooper, Ronald H.; Kerbey, Alan L.; Pask, Helen T.; Severson, David L.; Stansbie, David; Whitehouse, Susan (1975-10-01). "Regulation of mammalian pyruvate dehydrogenase". Molecular and Cellular Biochemistry. 9 (1): 27–53. doi:10.1007/BF01731731. ISSN 0300-8177.
- ^ Dimroth, P.; Kaim, G.; Matthey, U. (2000-01-01). "Crucial role of the membrane potential for ATP synthesis by F(1)F(o) ATP synthases". The Journal of Experimental Biology. 203 (Pt 1): 51–59. ISSN 0022-0949. PMID 10600673.
