Ethylene glycol (data page)
This page provides supplementary chemical data on ethylene glycol.
Material Safety Data Sheet[edit]
The handling of this chemical may incur notable safety precautions. It is highly recommended that you seek the Material Safety Datasheet (MSDS) for this chemical from a reliable source and follow its directions.
Structure and properties[edit]
| Structure and properties | |
|---|---|
| Index of refraction, nD | 1.4318 at 20°C |
| Abbe number | ? |
| Dielectric constant, εr [1] | 41.4 ε0 at 20 °C |
| Bond strength | ? |
| Bond length | ? |
| Bond angle | ? |
| Magnetic susceptibility | ? |
| Surface tension[1] | 47.99 dyn/cm at 25°C |
| Viscosity[1] | 16.1 mPa·s at 25°C |
Thermodynamic properties[edit]
| Phase behavior | |
|---|---|
| Triple point | 256 K (−17 °C), ? Pa |
| Critical point | 720 K (447 °C), 8.2 MPa |
| Standard enthalpy change of fusion, ΔfusH |
9.9 kJ/mol |
| Standard entropy change of fusion, ΔfusS |
38.2 J/(mol·K) |
| Standard enthalpy change of vaporization, ΔvapH |
65.6 kJ/mol |
| Standard entropy change of vaporization, ΔvapS |
? J/(mol·K) |
| Solid properties | |
| Standard enthalpy change of formation, ΔfH |
? kJ/mol |
| Standard molar entropy, S |
? J/(mol K) |
| Heat capacity, cp | ? J/(mol K) |
| Liquid properties | |
| Standard enthalpy change of formation, ΔfH |
−460 kJ/mol |
| Standard molar entropy, S |
166.9 J/(mol·K) |
| Heat capacity, cp | 149.5 J/(mol·K) |
| Gas properties | |
| Standard enthalpy change of formation, ΔfH |
−3955.4 kJ/mol |
| Standard molar entropy, S |
311.8 J/(mol·K) |
| Heat capacity, cp | 78 J/(mol·K) at 25 °C |
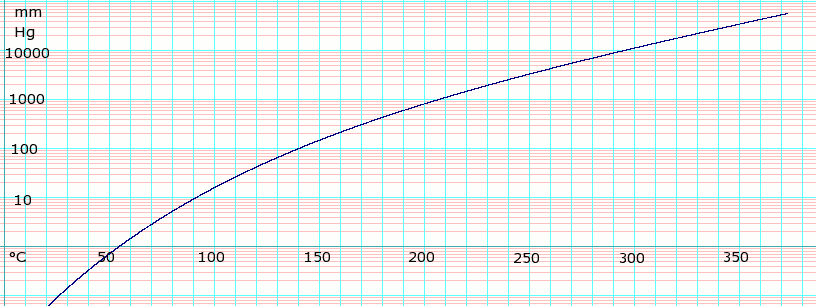
Vapor pressure of liquid[edit]
| P in mm Hg | 1 | 10 | 40 | 100 | 400 | 760 | |
| T in °C | 53.0 | 92.1 | 120.0 | 141.8 | 178.5 | 197.3 | |
Table data obtained from CRC Handbook of Chemistry and Physics, 44th ed.
Freezing point of aqueous solutions[edit]
| % ethylene glycol by volume | 5 | 10 | 15 | 20 | 25 | 30 | 35 | 40 | 45 | 50 | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Freezing point (°F) | −1.1 | −2.2 | −3.9 | −6.7 | −8.9 | −12.8 | −16.1 | −20.6 | −26.7 | −33.2 | |
| Specific gravity d90° | 1.004 | 1.006 | 1.012 | 1.017 | 1.020 | 1.024 | 1.028 | 1.032 | 1.037 | 1.040 | |
Table obtained from Lange's Handbook of Chemistry, 10th ed. Specific gravity is referenced to water at 15.6 °C.
See also "Typical Freezing and Boiling Points of Aqueous Solutions of DOWTHERM SR-1 and DOWTHERM-SR4000" (PDF). Dow Chemical. Archived from the original (PDF) on 27 September 2007. Retrieved 13 June 2007.
Distillation data[edit]
|
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Spectral data[edit]
| UV-Vis | |
|---|---|
| λmax | ? nm |
| Extinction coefficient, ε | ? |
| IR | |
| Major absorption bands | ? cm−1 |
| NMR | |
| Proton NMR | 3.3-3.7 ppm |
| Carbon-13 NMR | 62-65 ppm |
| Other NMR data | |
| MS | |
| Masses of main fragments |
|
This box:
- Except where noted otherwise, data relate to Standard temperature and pressure.
- Reliability of data general note.
References[edit]
- ^ a b c David R. Lide. Handbook of chemistry and physics CRC (2007), 87th ed.
- ^ "Temperature Dependent Properties. [PVP] Vapor pressure of ETHYLENE GLYCOL" (Queriable database). Pure Component Properties. Chemical Engineering Research Information Center. Retrieved 14 May 2007.
- ^ a b "Binary Vapor–Liquid Equilibrium Data" (Queriable database). Chemical Engineering Research Information Center. Retrieved 8 June 2007.
- Linstrom, Peter (1997). "NIST Standard Reference Database". National Institute of Standards and Technology. doi:10.18434/T4D303.
{{cite journal}}: Cite journal requires|journal=(help)

![{\displaystyle \log _{e}P[{\text{kPa}}]=-25.99771\log _{e}T[{\text{K}}]-{\frac {14768.57}{T[{\text{K}}]}}+191.4250+2.062331\times 10^{-05}\,(T[{\text{K}}])^{2}}](https://wikimedia.org/api/rest_v1/media/math/render/svg/181e9f3272538abcb78b427f83bb04d6f950ed1c)